For years, neuroscientists have been fixated on understanding how behavior and cognition arise from neural circuit activity.
To begin investigating these questions, scientists have been employing slow pharmacological and imprecise electrical stimulation techniques to control neural activity. However, with different classes of neurons communicating through vast, complex networks and fast electrical signals, it is essential to develop a method that can control neurons with precision to decode the function of neural circuit activity.
In 1979, Francis Crick proposed a novel solution: use light to control neurons (Boyden et al. 2005). Derived from this very idea and with the effort of many scientists, the revolutionary technique of optogenetics was born.
Optogenetics, a genetic method to turn select neurons on or off with light, was invented in 2005 by Karl Deisseroth and Edward Boyden (Boyden et al. 2005). Notably, their development of optogenetics began with a discovery by Peter Hegemann who successfully expressed the blue light-depolarizing opsin, channelrhodopsin-2 (ChR2), in cell culture (Nagel et al. 2003).
Expanding upon these findings, Deisseroth and Boyden virally expressed ChR2 in neurons, and, miraculously, they demonstrated for the first time that neurons could be activated using blue light (Boyden et al. 2005).
With millisecond precision, optogenetics enables the fast control of neuronal spiking. As a technique using both optics and genetics, the term opto-genetics was coined from their extraordinary findings.
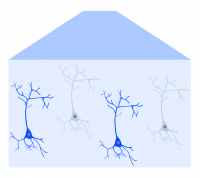
Optogenetic-expressing cells (in blue) are activated by blue light illumination.
How Does Optogenetics Work?
Optogenetics began with the discovery of opsins, such as ChR2. Opsins are light-sensitive channels that cause the depolarization or hyperpolarization of neurons through mechanisms such as the influx of ions or protein signaling cascades (Kim et al. 2017).
Opsins are sensitive to specific wavelengths of light, leading to the activation or inhibition of neural activity. For example, blue light (~470 nm) activates ChR2, causing an influx of Na+ ions and, in turn, depolarizing the neuron (Boyden et al. 2005). With viral expression and transgenic animal models, researchers can target optogenetic probes to genetically-defined neuron populations and across brain-wide projections (Kim et al. 2017).
Multi-disciplinary collaborations between neuroscientists, biologists, and engineers have led to the expansion of the optogenetic toolbox. Opsins have been discovered for manipulating neurons on or off at varying speeds and with different wavelengths of light (see Table 1). For example, Halorhdopsin, an inhibitory opsin, was found to turn off neurons, and red-activated opsins, such as JAWS, were developed to penetrate deeper into the brain (Kim et al. 2017).
| Optogenetic Construct | Excitation Wavelength | Function |
|---|---|---|
| ChR2 | 470nm | Activation |
| GtACR2 | 470nm | Inhibition |
| ArchT | 540nm | Inhibition |
| C1v1 | 560nm | Activation |
| NpHr | 590nm | Inhibition |
| bReaChES | 590nm | Activation |
| Chrimson | 590nm | Activation |
| ReaChR | 620nm | Activation |
| JAWS | 620nm | Inhibition |
What are the Applications of Optogenetics?
Optogenetics has benefited many scientific fields by providing the ability to control the activity of different cell-types with millisecond precision.
In neuroscience, optogenetics has enabled scientists to link neural circuits, behaviour, and function (Kim et al. 2017). Optogenetics can be performed both in vitro with electrophysiology and in a behaving animal with calcium imaging. Of great benefit to neuroscientists, optogenetics has been adapted for use in rodents, primates, C.elegans, drosophila, and zebrafish to study the neural correlates of cognition and behavior.
As a precise method to control cellular activity, optogenetics has impacted scientific fields beyond neuroscience with recent contributions to cell biology and cardiac research (Repina et al. 2017; Ferenczi et al. 2019).
From a clinical perspective, the application of optogenetics has begun to be used for exploring vision restoration and deep-brain stimulation in motor diseases (Towne & Thompson 2016).
With a causal method to analyze the function of neuron activity in real-time, optogenetics has advanced our understanding of the brain and may one day have significant clinical implications.
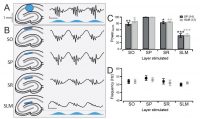
An example demonstrating the use of optogenetics with slice electrophysiology.
Next Post
What Equipment Do You Need to Perform Optogenetics?
——————————
Related Products for Optogenetics