The brain contains over a billion neurons, each with complex networks of connections. Patterns of neural activity are believed to generate specific aspects of behaviour and cognition, but how can this be addressed?
Current techniques, such as in vivo electrophysiology, can record neural activity with spike-timed precision, but they cannot localize the activity of large populations of individual cells or identify cell types
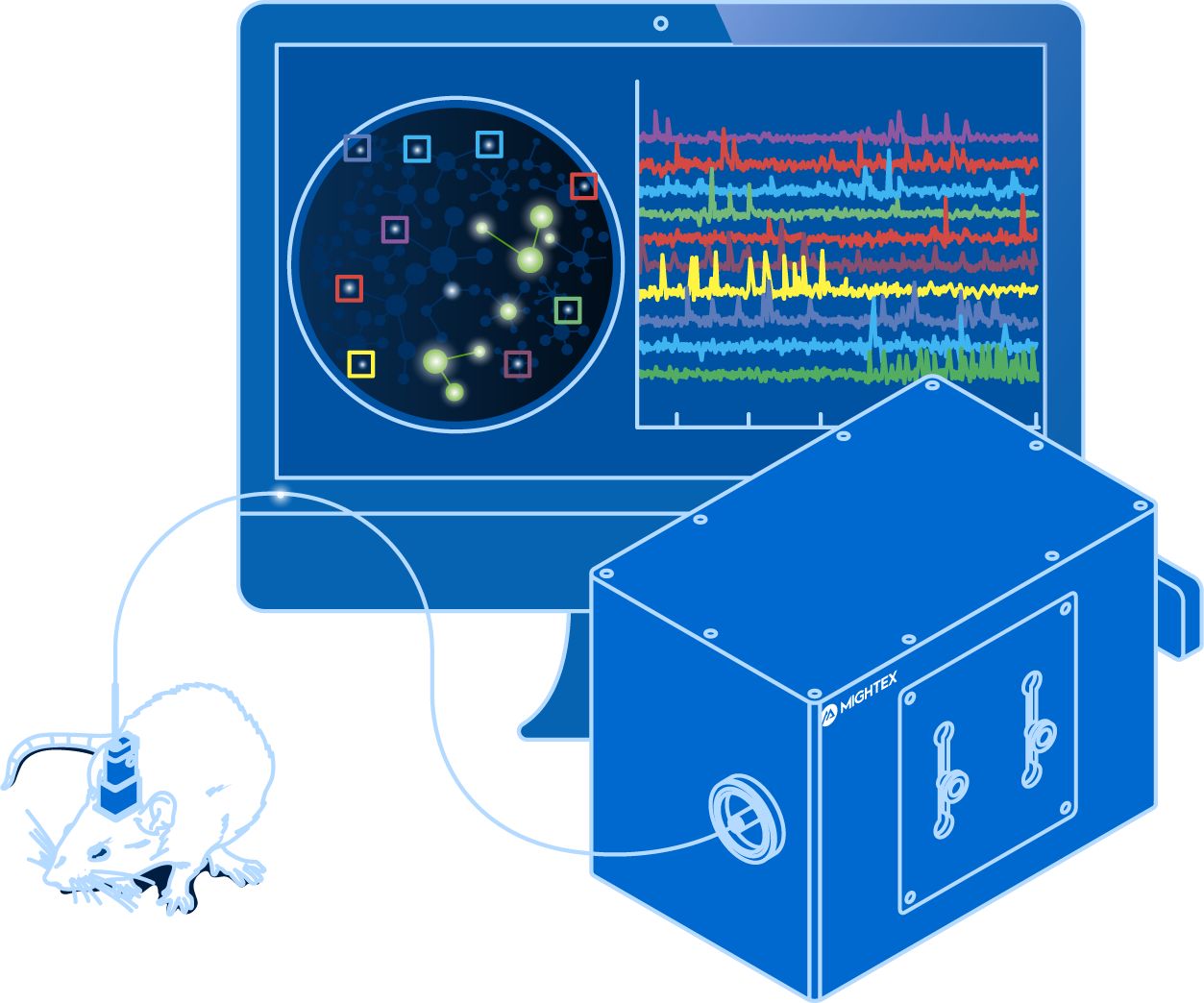
To decode patterns of neural activity, neuroscientists need to visualize hundreds of neurons simultaneously—not just an individual neuron—in freely-behaving animals. From a technology standpoint, this is no easy feat.
How can you visualize neural activity in vivo?
What is In Vivo Calcium Imaging?
Calcium imaging enables neuroscientists to visualize the activity of hundreds of individual neurons simultaneously using fluorescent activity sensors. Changes in fluorescence indicate fluctuations in intracellular calcium, which is an indirect indicator of neural activity (Grienberger & Konnerth, 2012).
The development of genetically encoded calcium indicators (GECIs) has enabled neuroscientists to study specific cell types (e.g., excitatory or inhibitory neurons).
GCaMP, a green fluorescent GECI, is commonly used in calcium imaging experiments since it has been optimized over many generations (currently GCaMP7) for speed, signal-to-noise ratio, expression, and changes in fluorescence (Dana et al. 2012). The calcium imaging toolbox is growing constantly with the development of new GECIs, such as RCaMP and XCaMP (Akerboom et al. 2013; Inoue et al. 2019).
Why Use In Vivo Calcium Imaging?
Recently, there has been a surge in the use of calcium imaging to study neural circuits in vivo. This popularity has led to both optimized biological methods and better optical technologies, allowing more neuroscientists to adopt calcium imaging.
Aside from popularity, why are neuroscientists drawn to calcium imaging?
In vivo calcium imaging provides the means to study specific populations of neurons within or across brain regions in freely-behaving animals. Thus, neuroscientists can investigate how neural activity may be linked to aspects of behaviour and cognition, connecting genetically-identified cells with function.
Importantly, behaviour and cognition cannot be characterized by an isolated firing pattern of neurons because they are learned or adapted over time. Calcium imaging can be used to track the activity of neurons over time and investigate how networks grow or change during learning. This is especially important for the longitudinal study of animal models. Neuroscientists can begin to understand the development of activity-related changes in these models and assess the long-term effects of pharmacological interventions.
With any new technique, there are associated pitfalls. As we’ve known for years, neural activity is associated with action potentials—changes in voltage across the cell membrane. Calcium imaging measures changes in intracellular calcium concentrations, providing an indirect indicator of neural activity. Compared to changes in voltage, fluctuations in calcium levels are much slower and may reflect a summation of signals rather than individual spikes (Wei et al. 2019). That is to say, the temporal resolution of calcium imaging may be limited for fast-spiking neurons, such as interneurons.
The gradual development of genetically encoded voltage indicators (GEVIs), designed to detect changes in voltage, is expected to overcome these limitations (Knopfel & Song, 2019). As with any new technology, widespread application of GEVIs will take time, requiring validation and optimization for use in vivo.
Despite this limitation, in vivo calcium imaging has been able to advance our understanding of how neural activity is linked to behaviour and cognition.
Next Post
What Do You Need to Perform In Vivo Calcium Imaging?
——————————
Related Products for In Vivo Calcium Imaging