Optogenetically eliciting precisely-timed action potentials in cerebellar purkinje cell axons
Kim Gruver & Alanna Watt
Department of Biology, McGill University
Optogenetics is a state-of-the-art tool for interrogating neural circuits, and has been shown to be well-suited for facilitating long-range circuit mapping. In the cerebellum, Purkinje cells serve as the sole output of the cerebellar cortex where they synapse on neurons in the deep cerebellar nuclei (DCN). To investigate the functional properties of this long-range synaptic connection, we sought to excite Purkinje cells with high spatiotemporal resolution while performing electrophysiology. To do this, we use the Mightex Polygon400E system to focally illuminate Purkinje cell axons (40 μm x 40 μm square) in transgenic mice expressing channelrhodopsin-2 (ChR2) with 470 nm light through a Mightex BioLED control module.
Since the Mightex Polygon400E system allows users to wield light with precise spatiotemporal control at a subcellular resolution, we set out to determine the optimal experimental conditions necessary to elicit time-locked single action potentials from Purkinje cell axonal stimulation. To optimize axonal photostimulation, we used whole-cell patch clamp recordings of ChR2-expressing Purkinje cells from acute cerebellar slices and photostimulated the Purkinje cell body or axon. We compared photostimulationin axons and cell bodies from individual Purkinje cells and found that a longer light pulse was necessary to elicit single action potentials from axons.
To explore axonal photostimulation conditions further, we exposed cells to ambient light prior to optogenetic activation and found that this caused both cell bodies and axons to be less sensitive to focused optogenetic activation. Of note, axons were more sensitive than soma were to ambient light, meaning that extra care needs to be taken when designing experiments to elicit spikes in axons, as we do here.
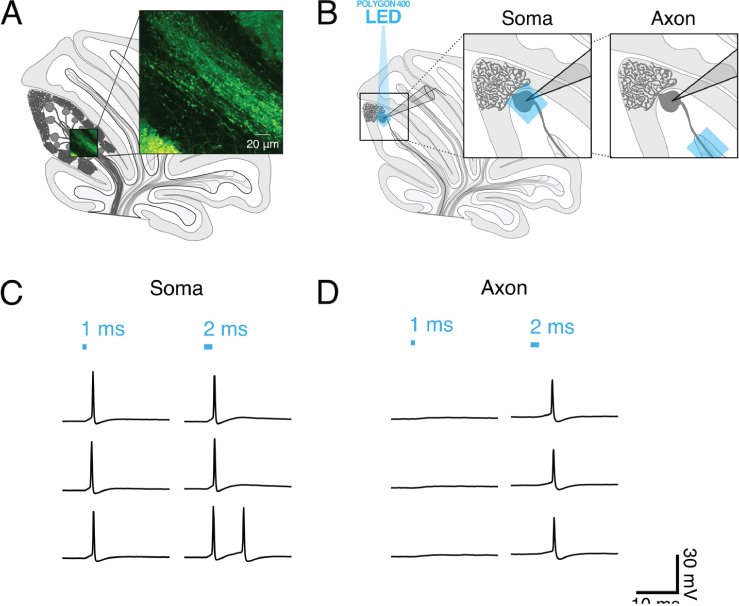
Figure 1. (A) Schematic of sagittal cerebellar slice. Inset is a maximal intensity projection of abtwo-photon stack showing ChR2(H134R)-EYFP expression (green) in axons in the white matter. (B) Recording configuration. Insets show the focal region of photostimulation (blue square) and somatic recording electrode. (C and D) Representative current-clamp traces of optically-evoked action potentials evoked following somatic (C) and axonal (D) stimulation. Blue bars above trace indicate onset and duration of light pulse.
Finally, using our empirically-determined optimal axonal photostimulation parameters, we confirm that these stimulation parameters are functional by eliciting time-locked IPSCs in DCN neurons with minimal jitter. The precise spatiotemporal control afforded to us by the Mightex Polygon400E system has thus allowed us to identify the optimal experimental conditions necessary to optogenetically explore Purkinje cell connectivity in the cerebellum.
Since optogenetic photostimulation of Purkinje cell axons is sufficient to elicit activity in postsynaptic DCN neurons, we will continue exploring the properties of functional connectivity at the Purkinje cell—DCN synapse. Using the Polygon400E system, going forward we will perform spatial mapping of these connections in the cerebellum, uncovering functional projection patterns within this brain region to better understand how information transfer occurs within the cerebellum.
Author: Kim Gruver, McGill University
Bio: Kim Gruver graduated with honors from Cornell before moving on to McGill University to pursue her PhD in the lab of Dr. Alanna Watt. Kim is deeply curious about how the cerebellum works. Her research uses optogenetics and electrophysiology to plumb the depths of the cerebellar microcircuit and contribute to our understanding of this fascinating brain structure.



