Induction of CA1 Theta-Nested Gamma Oscillations Using Layer-Specific Optogenetic Stimulation
James Butler
Department of Neurosciences, University of Cambridge
Gamma oscillations (30 – 120 Hz) are found across the mammalian brain and are thought to be important for many cognitive functions including working memory and perception (Buzsaki & Wang, 2012). Area CA1 of the hippocampus exhibits two prominent gamma oscillations which originate from its afferent regions, CA3 and the medial entorhinal cortex (mEC; Colgin et al., 2009). More recently a third gamma oscillation has been described which is likely to be independent of these afferent regions and instead generated intrinsically within CA1 (Schomburg et al., 2014).
To investigate the ability of CA1 to generate its own gamma oscillations in vitro we crossed +/+ CaMKII-α-Cre mice on a C57BL/6 background with +/+ LoxPChR2(H134R)-EYFP (Ai32) mice on a 129S6 background. This resulted in first generation offspring which expressed the optogenetic activator, channelrhodopsin-2 (ChR2), in pyramidal neurons. Acute hippocampal slices were then prepared from these mice and mounted on a 64-channel multielectrode array to allow for electrophysiological recordings of the CA1 region. Sinusoidally-modulated blue light stimulation of the CA1 region induced robust theta-nested gamma oscillations (Figure 1A). These gamma oscillations occurred in both the stratum pyramidale (SP) and stratum lacunosummoleculare (SLM), but not the stratum radiatum (SR) or stratum oriens (SO), of CA1.
In the transgenic model used here, all pyramidal neurons were expressing ChR2, not just the CA1 pyramidal neurons that are the focus of this study. Therefore, stimulating the entire CA1 region does not distinguish whether it is stimulation of the CA1 pyramidal neurons themselves, or stimulation of the terminal afferent projections in CA1, that is involved in generating the gamma oscillations. Thankfully, the organisation of the CA1 region is such that the afferent inputs terminate in layers separate from the pyramidal neuron somatic layer, the SP. Thus, it was possible to test for the importance of afferent input versus pyramidal neuron stimulation for the gamma oscillations by restricting the light to the individual layers of CA1. To do this a digital micromirror device (Polygon400, Mightex) was used which was capable of focusing the light into a narrow band less than 100 μM in height, therefore restricting stimulation to specific layers of CA1 (Figure 1B).
Light stimulation of all four layers was found to induce gamma oscillations in the SP layer. Light stimulation caused gamma oscillations of the highest magnitude when the light was focused over the SP layer itself (Figure 1B and 1C, middle panel). Light stimulation of the adjacent SO or SR layers induced gamma oscillations with a slightly reduced power of 78 ± 5% and 83 ± 4% respectively compared to stimulation of SP (p = 0.0019 and 0.0055 respectively, n = 14; Figure 1B and C). When the SLM was stimulated, the location of EC projections and also the layer furthest from the perisomatic layer, the oscillations decreased in power further to 42 ± 6% (p = 0.0001, n = 14; Figure 1B and 1C). Moreover, these changes in power also occurred in the gamma oscillations recorded from the SLM (SO stimulation: 87 ± 5%, SR: 85 ± 2%, SLM: 44 ± 4%, p = 0.0161, 0.0008, and 0.00001 respectively, n = 12; Figure 1B). Despite these large changes in power, the frequency of the gamma oscillations remained consistent across all conditions tested (Figure 1D).
Therefore, stimulation of the CA1 pyramidal neuron somas in SP induced the largest gamma oscillations and stimulating the area of the afferent inputs into CA1 induced a much smaller oscillation with a comparable frequency. This suggests that the gamma oscillations are generated intrinsically independent of the afferent inputs. Our further findings on optogenetically-induced CA1 theta-nested gamma oscillations can be found in Butler et al. (2016).
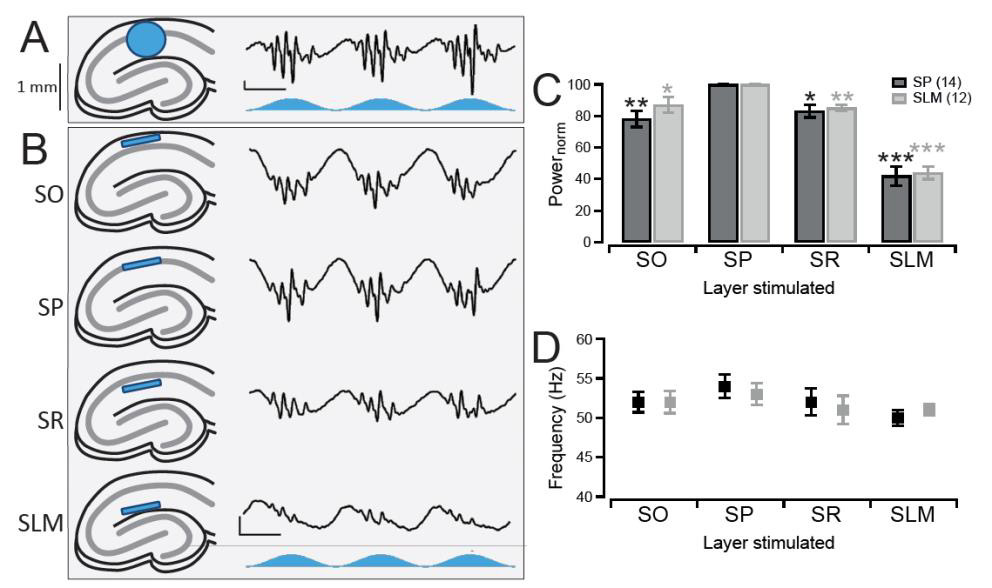
Figure 1. Optogenetic induction of CA1 gamma oscillations using stratum pyramidale stimulation
A, Theta stimulation of CA1 in acute hippocampal slices (left, blue circle represents the area of light stimulation) where all pyramidal neurons are expressing channelrhodopsin-2 (ChR2) resulted in the generation of theta-nested gamma oscillations as seen in vivo (right, blue trace represents the temporal light stimulation). Vertical Scale bar, 50 μV. Horizontal scale bar, 100 ms. B, Stimulation of the entirety of CA1 however did not distinguish between stimulation of the CA1 pyramidal neurons in the cell body layer (stratum pyramidale, SP) and stimulation of the dendrites of inputs into CA1 in the other layers (stratum oriens (SO), stratum radiatum (SR), and stratum lacunosum-moleculare (SLM)). Therefore a digital micro-mirror device was used to focus a narrow beam of light 100 μM in height on only a specific layer within the CA1 region (left, blue strip). Gamma oscillations could still be induced when light was focused on all four layers (right), with stimulation of SP generating the largest amplitude oscillations (second row). Same scale bar as in A. C, Gamma oscillations occurred in both the SP (black) and SLM (grey) layers, with the largest power for the oscillations in both regions being when the light was focused over the SP layer. The values are normalised to when the light was focused on the SP layer. N = 14 for SP and n = 12 for SLM recordings.*,**, and *** denotes p < 0.0167, 0.0033 and 0.0003 respectively using a paired t-test with Bonferroni corrections to compare that layer to when the light was focused over SP. D, Same as in C but for the average frequency of the oscillations. There were no significant differences in the frequency between SP stimulation and stimulation of any of the other layers when using a paired t-test with Bonferroni corrections (p-value threshold = 0.0167). Error bars represent standard error of the mean.


