Published on 2023/06/26 Research powered by Mightex’s OASIS Implant 


Check out this recent work by Sandra Poulson using the OASIS Implant for optogenetics. Sandra recently presented her work at the United States Association for the Study of Pain 2023 in Durham, NC. We are excited to see more to come from Sandra using the OASIS Implant for her research!
Sandra Jaramillo Poulson is a PhD candidate in Loren Martin’s Laboratory at the University of Toronto Mississauga. Sandra has a BSc in Biology and MA in Scientific Education from the University of Texas, San Antonio as well as a MSc in Behavioural Psychology from the University of Toronto Mississauga. Sandra presented this work at the United States Association for the Study of Pain 2023 in Durham, NC.
Introduction
Expectations of enhanced pain and injury, or nocebo, can arise from environmental cues such as surroundings, even cues from social partners. Interaction with a cagemate in pain typically induces mechanical sensitivity in observer mice, but the neuromolecular and neural circuit mechanisms that drive this enhanced pain are unknown. One neuropeptide of particular interest in this context is cholecystokinin (CCK). Previously research from Sandra Poulson and Loren Martin’s lab at the University of Toronto, Mississauga showed that systemic antagonism of cholecystokinin (CCK) receptors blocks enhanced pain in mice provoked by observing a social partner expressing overt pain (reference). However, the role of CCK in the social mediation of pain, injury, and nocebo is not fully elucidated.
Methods & Findings
As such, Sandra and colleagues sought to determine the function of cholecystokinin receptors in pain enhancement triggered by social cues from the environment. Description of the research project Based on their finding of increased neural activation (immunohistochemistry for c-Fos) in the lateral periaqueductal grey (PAG) of observer mice, they cannulated mice (C57BL/6, same sex cagemate pairs of both sexes, 8-9 weeks old) above the lateral PAG bilaterally. After waiting 1 week for recovery, they then applied bilateral drug microinfusions to the lateral PAG to test the effects on behavior. They found that application of proglumide, a cholecystokinin antagonist, to the lateral PAG diminishes enhanced pain when observing a cagemate in pain. Additionally, they found that application of cholecystokinin to the lateral PAG enhances paw flicking and licking responses on the hot plate and radiant heat test.
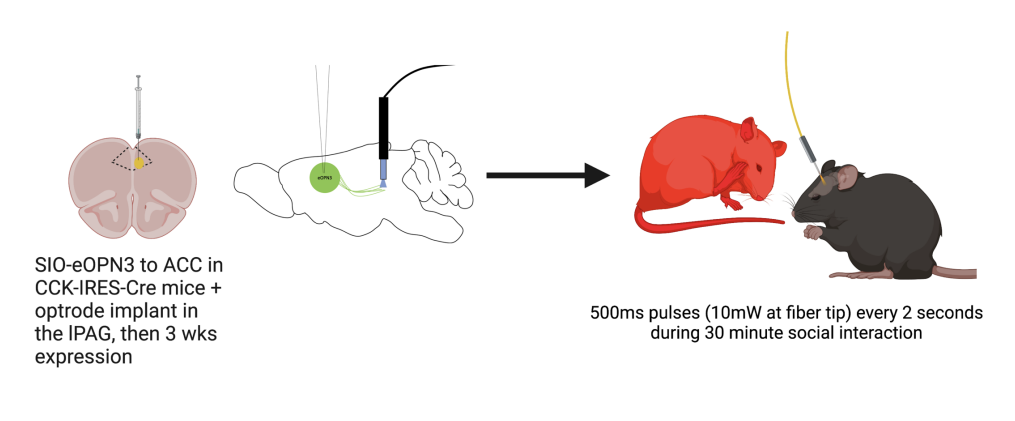
Figure 1. Schematic illustration of virally-mediated eOPN3 expression and optrode placement in CCK-IRES-Cre mice. Optrode focused on ACC terminals in iPAG. Stimulation conducted using 500ms pules of 10mW 470nm illumination using the OASIS Implant. Stimulation duration of 2s throughtout 30min social task.
In order to determine the effects of CCK silencing in a projection specific manner, Poulson and colleagues employed optogenetics. They used a virally -mediated approach, introducing eOPN3 to optogenetically silence the CCK projections of the ACC at the lPAG in the observer mouse. They found that eOPN3-induced inhibition of ACC-iPAG CCK neruons disrupted enhanced pain due to social interaction. In order to conduct these experiments, Poulson and colleagues used the Mightex OASIS Implant along with a 470nm collimated LED light source and a 200nm patchcord to silence CCK projections using 500ms pulses of 10mW of light at the fiber tip every 2 seconds over a 30 minute session. Optical inhibition was followed by manual pain tests to examine pain sensitivity.
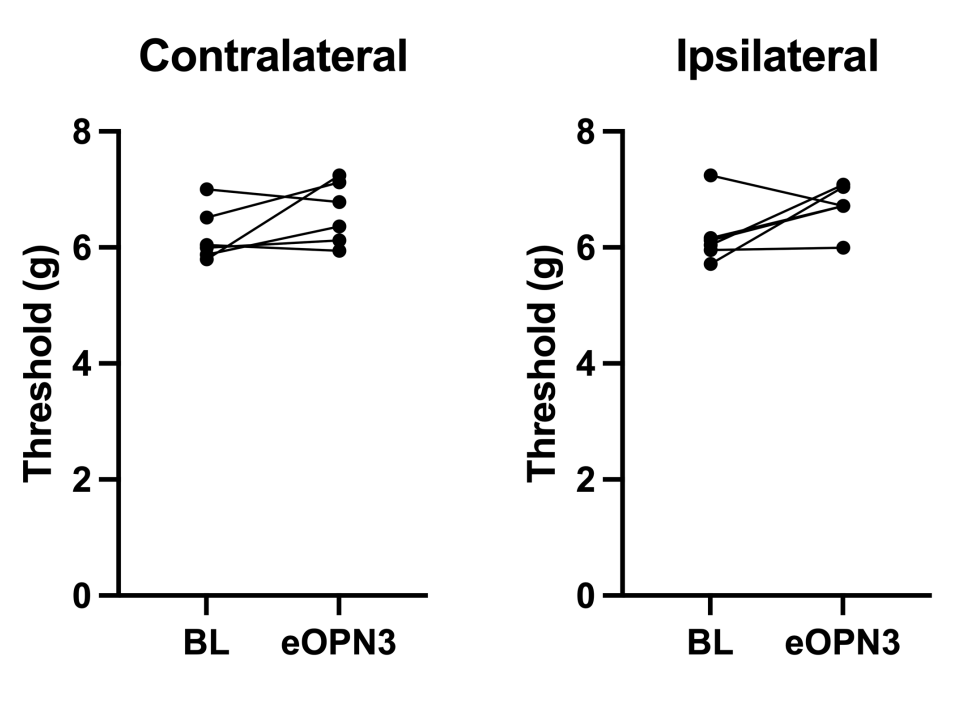
Figure 2. Preliminary data demonstrating eOPN3-mediated inhibition of socially mediated pain. figures show contralateral and ipsilateral pain threshold responses following silencing of ACC-iPAG CCK-expressing neurons.
Findings and Conclusion
These data indicate that cholecystokinin receptors in the lateral PAG are involved in descending facilitation of the nocebo response when mice observe cagemates in pain. These findings build a more neuroanatomically precise understanding of the role of cholecystokinin in pain facilitation due to social context and the neural mechanisms underlying environmental and social-communicative enhancement of pain. Sandra currently uses the Mightex OASIS Implant for fiber photometry and optogenetics experiments. To learn more about how you can use the OASIS Implant for your research, read more here.
Catherine Thomas, PhD Senior Liaison and Development Scientist at Mightex



