Published on 2024/02/15 Research powered by Mightex’s Polygon1000 

Vu, M. A. T., Brown, E. H., Wen, M. J., Noggle, C. A., Zhang, Z., Monk, K. J., … & Howe, M. W, Targeted micro-fiber arrays for measuring and manipulating localized multi-scale neural dynamics over large, deep brain volumes during behavior. Neuron, (2024).


The latest publication from Mark Howe’s lab at Boston University showcases a novel optical approach to elucidate neuronal dynamics at large spatial and temporal dimensions over 3D volumes. The group designed a new implantable micro-fiber array technique that allows recording and optogenetic manipulation of up to 100 locations simultaneously in head-fixed and freely moving animals.
Specifically in the head-fixed approach, the authors implemented this technique to investigate the spatial distribution and functional implications of neuromodulatory signals within the striatum, a crucial brain region implicated in motor control, perception, and learning. They aimed to elucidate the impact of large-scale neuromodulator release on behavior, recognizing the heterogeneous expression patterns across three-dimensional volumes in the striatum. However, the precise assessment of these effects demanded tailored manipulations that could match the spatial and temporal properties of endogenous release, a feat unattainable with conventional optogenetic techniques employing large-diameter optical fibers.
To address this limitation, the researchers integrated Mightex’s Polygon1000-DL digital micromirror device (DMD) into their microscopy setup, allowing for targeted illumination of the individual microarray fibers with laser light at 465 nm wavelength (Fig. 1A). Leveraging this setup, they expressed the excitatory opsin channelrhodopsin-2 (ChR2) in midbrain dopamine (DA) neurons and the red DA sensor rDAm3.041 across the striatum, followed by implantation of the fiber array to target specific locations within the striatum volume (Fig. 1B).
Characterization of the system revealed the capability to deliver light spots precisely to individual optical fibers without leakage into neighboring fibers (Fig. 1D). Calibration of light spot power at the objective lens ensured a power density at the fiber tip sufficient to activate ChR2-expressing axons within a defined axial volume (Fig. 1C). Importantly, this activation volume was notably smaller than that achieved through conventional optical fiber setups, underscoring the enhanced spatial precision of the DMD-based approach.
Subsequent experiments involved stimulation of individual fibers while measuring dopamine release across neighboring sites. Significant transient increases in stimulation-evoked DA release were observed at sites proximal to the targeted fiber, with a sharp decline in signal intensity with increasing distance in all three dimensions (Fig. 1E, F, G, H). Importantly, these observations remained consistent across different experimental conditions, indicating that the observed DA release spread was not attributable to direct light propagation but likely stemmed from the branching morphology of DA axons.
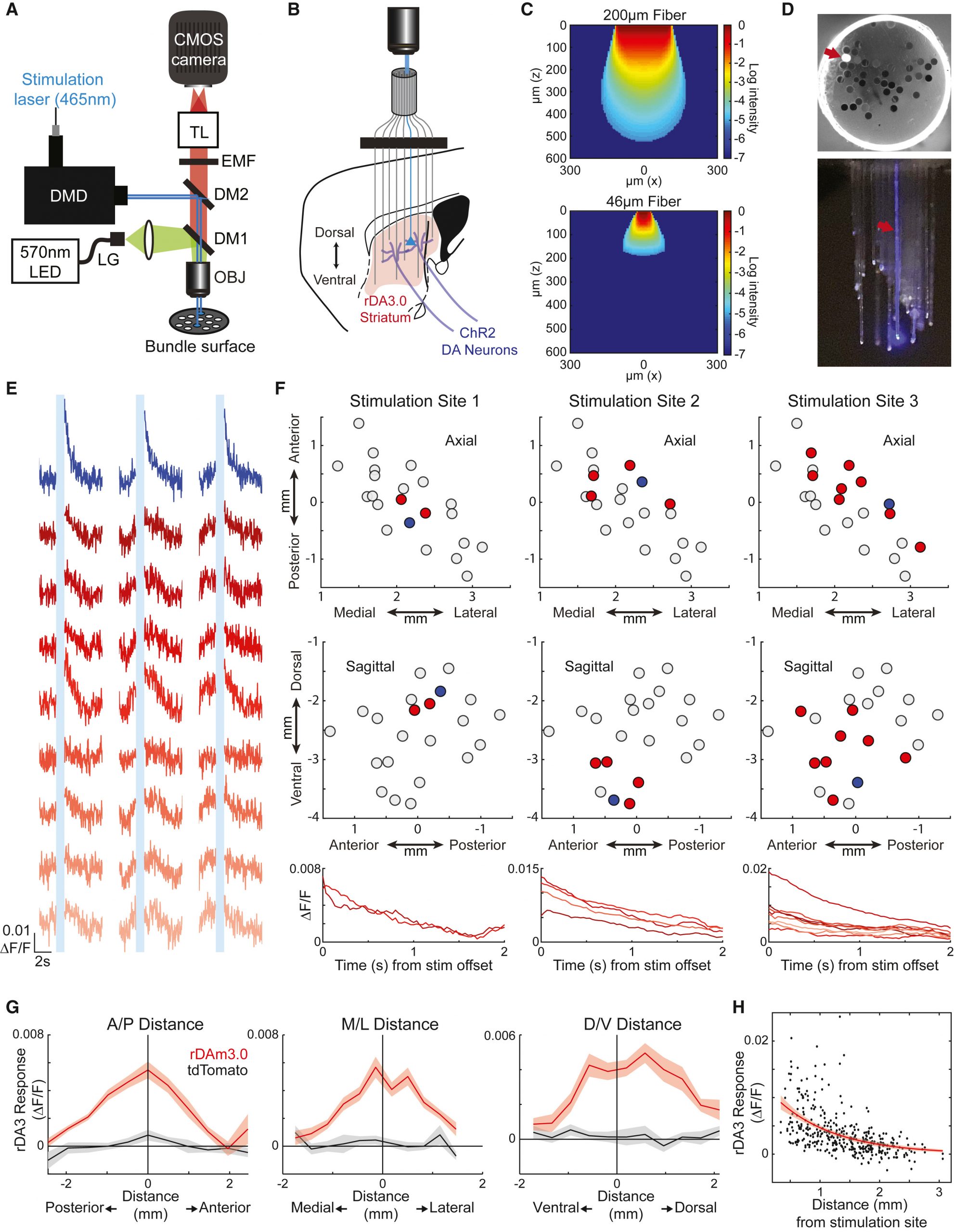
Figure 1: Microscopy setup for targeting individual fibers in implanted microarray for localized optogenetic manipulation over a large brain volume in head-fixed animals.
Moreover, the researchers demonstrated the applicability of their methodology in studying behavior by selectively stimulating D1-expressing spiny projection neurons (dSPNs) in distinct regions of the striatum (Fig. 2). Using live video recordings and computational tracking, they quantified changes in orofacial movements elicited by optogenetic stimulation. By targeting specific regions within the ventral and dorsal striatum, they could elicit region-specific changes in orofacial movements, highlighting the capacity of their approach to precisely modulate behavior by targeting discrete neural circuits.
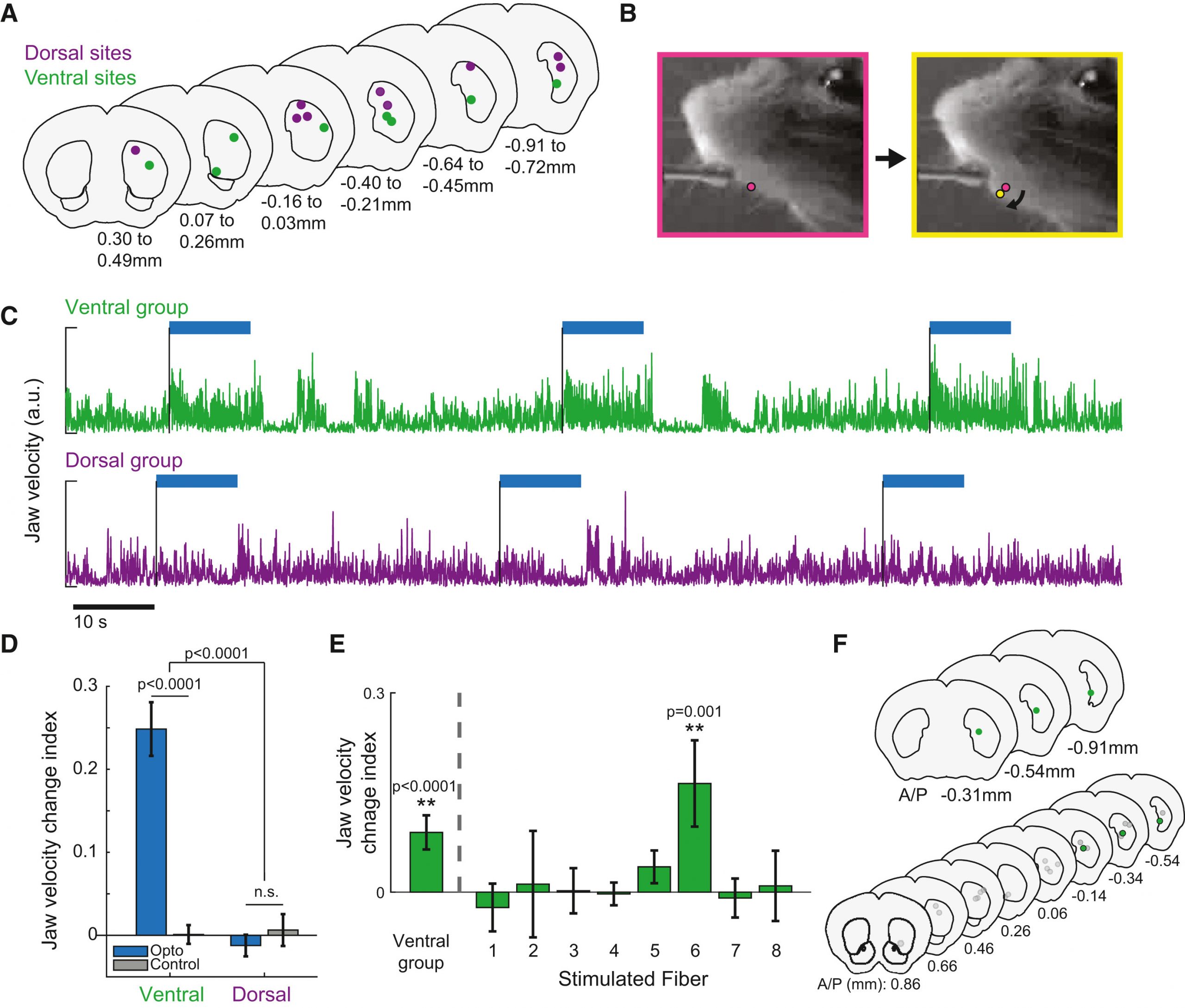
Figure 2: Localized optogenetic manipulation of dorsal and ventral regions in the striatum to investigate region-specific effects on facial movements.
In conclusion, the study presents a comprehensive methodology employing advanced optogenetic and imaging techniques to achieve precise control and manipulation of neuromodulatory signals within the striatum. Their findings not only contribute to a deeper understanding of the spatial dynamics of neuromodulation but also offer valuable insights into the functional organization of neural circuits underlying behavior.
Francisco Rodrigues, PhD Manager, Customer Solutions at Mightex
To read the full publication, please click here.



