Worldwide OASIS Implant Customer Examples
The OASIS Implant has been used by labs all over the world for a wide range of research applications. Scroll through the list below to see examples of how some of our customers are using the OASIS Implant in their research.
In Vivo Freely-Behaving Imaging of Intracellular Signalling Pathways of Hippocampus-Dependent Memory.
Combining genetic, pharmacological and optogenetic manipulation of intracellular signalling with in vivo live calcium imaging of hippocampal neurons in freely behaving mice using Mightex OASIS Implant micro-endoscopy system, elucidates dynamic intracellular signaling mechanisms that regulate neuronal circuit activity during hippocampus-dependent memory.
The video shows in vivo GCaMP6 fluorescence imaging in hippocampal CA1 pyramidal neurons with the Mightex OASIS Implant micro-endoscopy system
Courtesy Jayant Rai & Dr. Kenichi Okamoto, University of Toronto, Canada
In Vivo Calcium Imaging in the Cingulate Cortex During Social Interaction in Freely-Behaving Mice
The cingulate cortex (CC) is a key brain region in the limbic system that coordinates actions and motivational behaviors. Somatostatin-expressing GABAergic neurons in the cingulate cortex (CCSST) can provide powerful inhibition to the CC circuitry through high basal firing activity and synchronized firing.
The video shows Ca2+ activity of somatostation-expressing cingulate cortex neurons during social interaction collected using the Mightex OASIS Implant system.
Courtesy Huanhuan Li & Dr. Geoffrey Lau, City University of Hong Kong, Hong Kong
Deep-Brain Calcium Imaging in Freely-Behaving Mice
In this video, a freely-behaving mouse is connected to the OASIS Implant via an imaging fiber (on the right). The OASIS Implant is imaging GCaMP signals from the striatum (on the left) during the animal’s behaviour
Courtesy Dr. Alexxai Kravitz, Washington University in St. Louis
Cortical Region Optogenetics in Freely-Behaving Mice
The video on the right features the OASIS Implant being used in combination with the Polygon400 to deliver targeted optogenetic stimulation to the cortex of a freely behaving mouse. When the optogenetic stimulation is localized within a specific region in the lower right quadrant the mouse responded with coordinated motor movements resembling a natural behavior. However, when the optogenetic stimulation is localized within a region in the upper right quadrant, the mouse exhibited a different behavior.
Courtesy Prof Zhigang He and Noaf Alwahab, Harvard Medical School
Worldwide Polygon Customer Examples
The Polygon is being used by 450+ labs all over the world for a wide range of research applications. Scroll through the list below to see examples of how some of our customers are using the Polygon in their research.
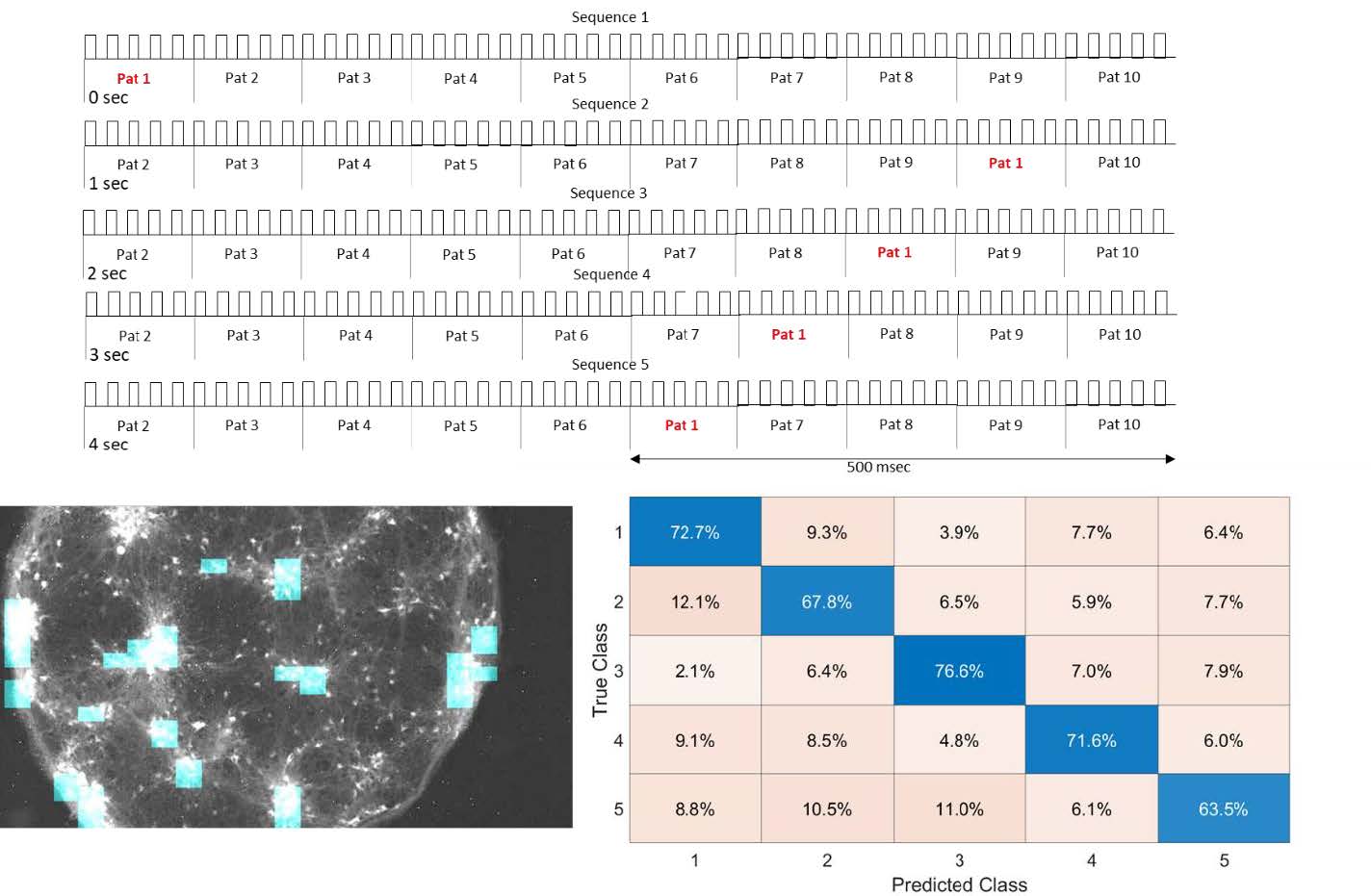
Neural Computation in Cortical Cultures
Confined neural networks in vitro with optical interface can be used to classify spatiotemporal information.
Patterned optogenetic stimulation to cultured ChR-expressing dissociated neurons resulted in suppression of population bursts. Strong correlation between outputs and input patterns that indicates processing of spatial information. Further analysis showed that the cortical network in vitro could classify and store information of spatiotemporal patterns up to 500 msec
By applying distributed optical stimulation patterns in the network, population bursts can be suppressed and simultaneously used to classify spatiotemporal information in the output neurons. These findings outline a paradigm of reservoir computing with living neural network which can be used to study spatiotemporal information in neural networks.
Courtesy Zubayer Ibne Ferdous & Dr. Yvgeny Berdichevsky, LeHigh University, USA
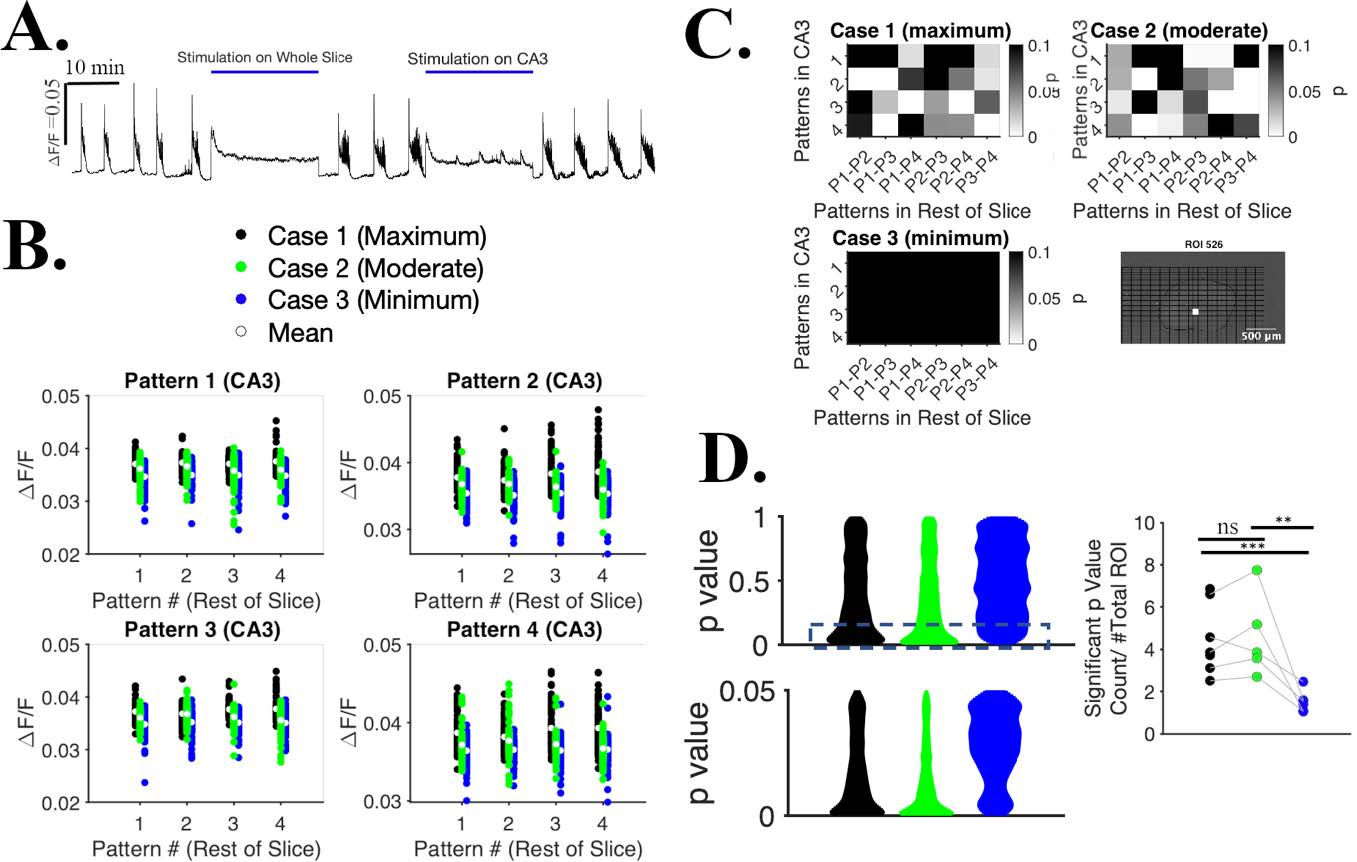
In-vitro Seizure Suppression by Patterned Optogenetic Stimulation while Allowing Information Processing in Hippocampus
Development of an effective method for suppressing seizures in such a way that activity in stimulated region is not completely shut off during the intervention. This allows stimulated area to participate in information processing and exchange of information with other brain regions, and may be a more gentle method of suppressing seizures than existing protocols.
Selective patterned optogenetic CA3 stimulation with ChR2 resulted reduction of seizure rate is lower and duration while not tetanizing the cells during stimulation and retaining the ability process other inputs during stimulation.
Courtesy Md Joynal Abedin & Dr. Yvgeny Berdichevsky, LeHigh University, USA
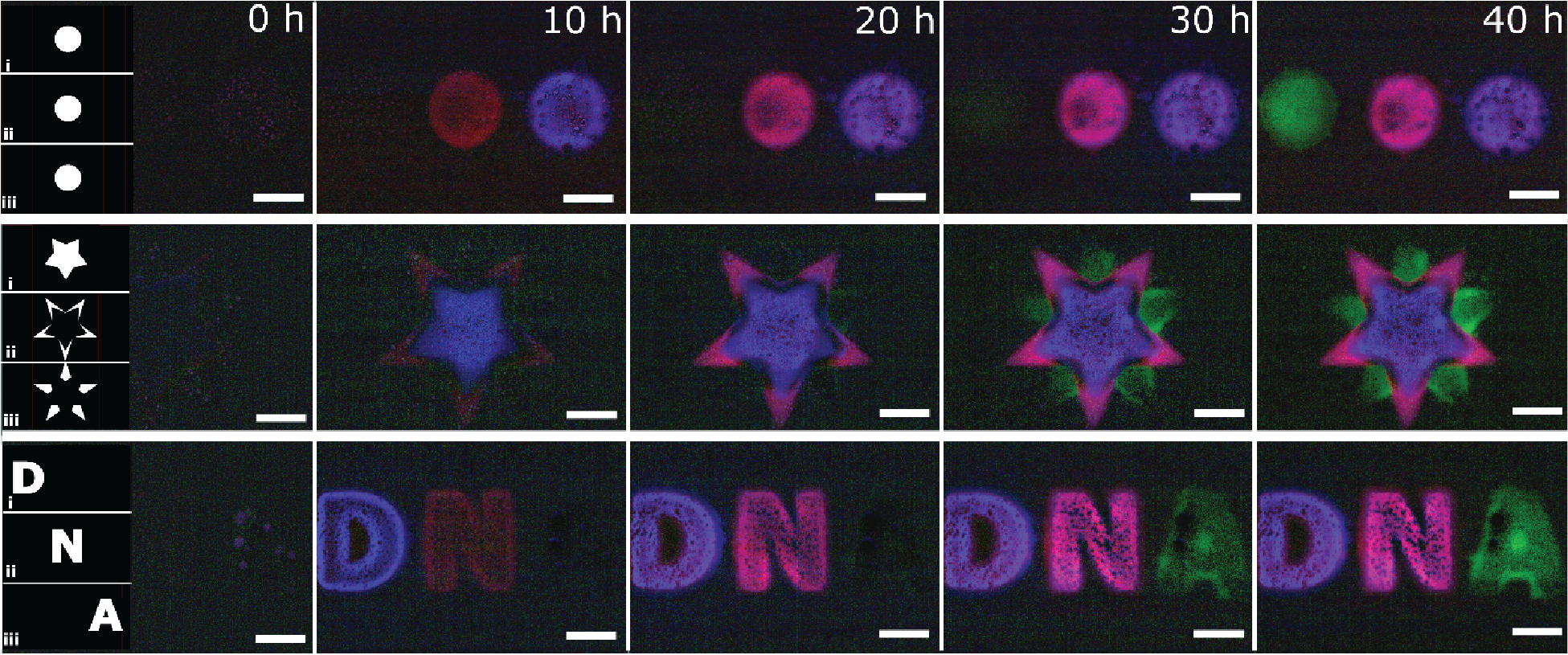
Photopatterning of Multi-Domain DNA Hydrogels
Fluorescent signals caused by the release of DNA from three 3-domain hydrogels that have unique embedded DNA within each domain.
Each row depicts the time-lapse for each multi-domain hydrogel, while each column represents the time of each photo. The blue domain activates first, followed by the red and then the green domain.
The digital masks uploaded to the DMD are shown in the left-most panel – each mask (row 1, 2, and 3) is used for the patterning of a hydrogel section with unique DNA molecules embedded within: Mask 1 contains red stage-1 DNA (released first), mask 2 contains blue stage-2 DNA (released second), and mask 3 contains green stage-3 DNA (released third). Scale bar is 200 µm.
Courtesy Misha Rubanov & Dr. Rebecca Schulman, Johns Hopkins University, USA
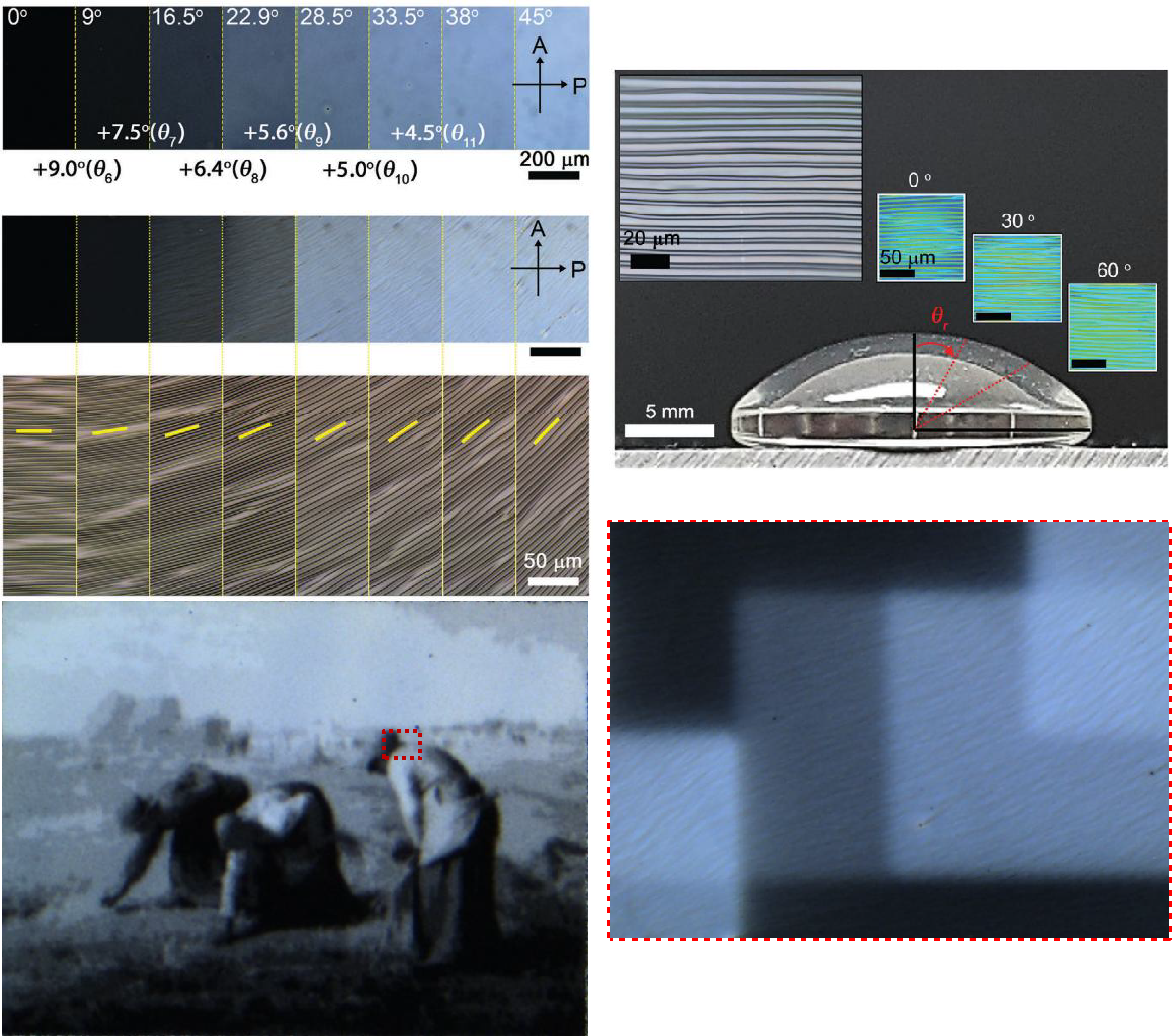
Photopatterning with mercaptopropylsilatrane and the Polygon 1000
By projecting different patterns, it is possible to control where molecules are attached, and so obtain monolayers of arbitrary shape. This results in monolayers with partial coverage – by breaking down a grayscale image into a series of different black and white images, researchers can individually control the exposure time at each pixel, and correspondingly tune the density of deposited dye molecules at each position.
The images are: 1) “Girl with a Pearl Earring” by Johannes Vermeer, 2) black-and-white version of “The Scream” by Edvard Munch, 3) “The Cardsharps” by Michelangelo Merisi da Caravaggio, and 4) the seal of Marquette University. Each image in the figure has an area of only 0.48 mm^2.
Courtesy Mark Mitmoen & Dr. Ofer Kedem, Marquette University, USA

Patterned Optogenetic Stimulation to Mimic Odors
The Mightex Polygon used to mimic “odors” by optogenetically activating spatio-temporal patterns in the early olfactory system of the mouse. Presentation of even monomolecular odorants already results in complex patterns of neural activity with unique but rigid spatiotemporal properties.
The Mightex Polygon provides full parametric control over both spatial and temporal properties of the optogenetic “odors” by precisely activating light spots over the dorsal olfactory bulb.
Courtesy Pedro Herrero-Vidal, Dr. Dima Rinberg, & Dr. Cristina Savin, New York University, USA
Pixelated Wrinkles Using Patterned Optical Phase Retardation
The Mightex’s Polygon1000 model used to form pixelated wrinkles on various surfaces. In addition, the continuous exposure technology of the Mightex Polygon has made it possible to locally expose the surface in conjunction with Nikon’s microscope software.
The optics based on a digital micro mirror technology (Polygon) assembles wrinkle pixels of a notably small dimension over a large area at fast fabrication speed. Furthermore, these pixelated wrinkles can be formed on curved geometries
Courtesy Kitae Kim & Dr. Jun-Hee Na, Chungnam National University, Korea
Patterned Optogenetics and Zebrafish Spinal Cord Organization
In vivo optogenetics and electrophysiology used to map connectivity of a major inhibitory population: V1 (En1+) neurons in larval zebrafish. Using the unique illumination patterns available with the Mightex Polygon, a 16×16 grid projected spanning approximately one spinal segment and sequentially activated 0-6 V1 neurons at a time. By translating this grid illumination to different spinal segments rostrally and caudally, connectivity tested from V1 neurons along significant lengths of the spinal cord.
Courtesy Dr. Mohini Sengupta & Dr. Martha Bagnall, WUSTL, USA
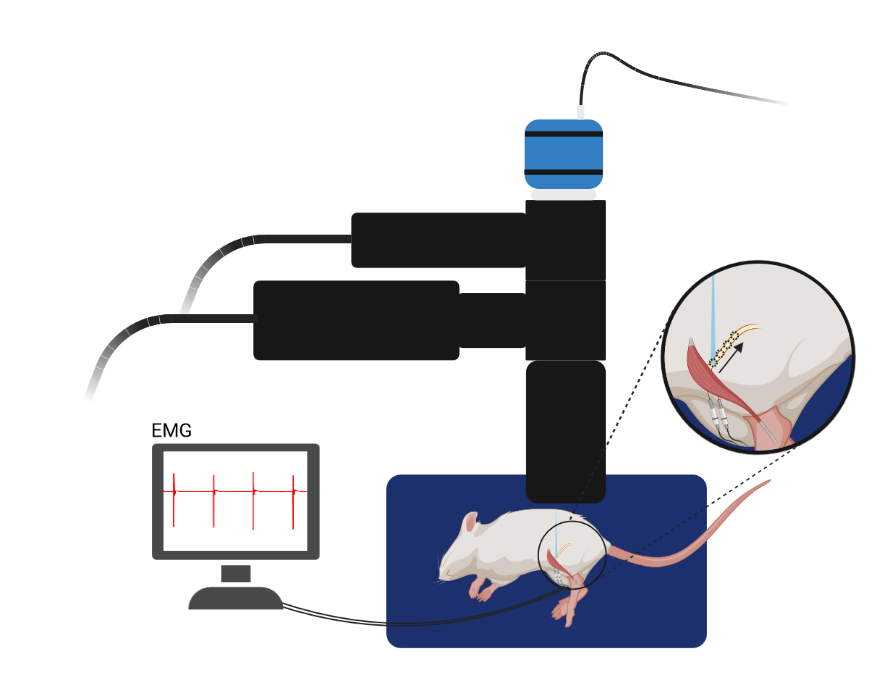
Patterned Optogenetics and Characterization of Muscular Responses
Polygon system used to stimulate the peroneal nerve in small segments starting from the distal muscle insertion moving proximally towards the sciatic nerve in a rat expressing Channelrhodopsin-2 (ChR2) in the peroneal nerve after an intramuscular virus injection in the Tibialis Anterior (TA) muscle. Simultaneous recording of muscle response to the optical stimulation in the TA muscle with intramuscular electromyography.
Courtesy Emma Moravec & Dr. Jordan Williams, Marquette University, USA
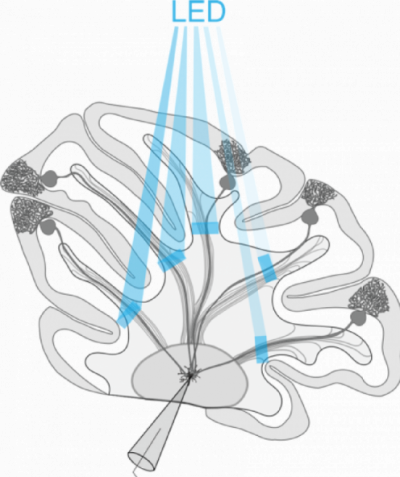
Patterned Optogenetics and Cerebellar Functional Connectivity
Patterned optogenetics stimulation of transgenically expressed ChR2 in Purkinje cells combined with electrophysiological recordings to evaluate patterns of connectivity between Purkinje cells and cerebellar nuclei neurons.
The Polygon is used to sequentially stimulate different cerebellar lobules in quick succession to assess the presence of a functional connection. These data are then used to characterize how different kinds of information are integrated within the cerebellum, and how these patterns may be implicated in behavior.
Courtesy Kim Gruver & Dr. Alanna Watt, McGill University, Canada
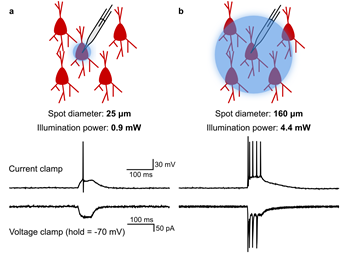
Single-Cell Resolution Optogenetic Spiking
(a) Illustration of highly targeted optical stimulation of a single ChR2-mCherry expressing neuron in the mouse somatosensory cortex. Using a small (25 μm diameter), low-powered (3 mW) spot of illumination centred on the target cell, action potentials could be induced in current clamp (top trace), without any indication in voltage clamp of post-synaptic currents caused by spiking in other neurons (bottom trace).
(b) Illustration of illumination with a large (125 μm diameter), high-powered (15 mW) spot. Multiple spikes were induced in current clamp, and voltage clamp traces showed evidence of post-synaptic currents caused by spiking in other neurons.
Courtesy Matthew Tran & Dr Blake Richards, University of Toronto, Canada.
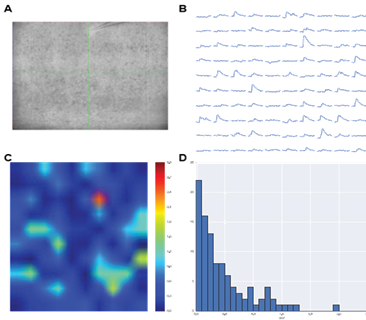
ChR2 Assisted Circuit Mapping (CRACM)
(A) Image of an acute brain slice prepared from a Thy1-ChR2-EYFP mouse with ChR2 expression in L5 pyramidal neurons. Whole-cell patch clamp recording from a L2/3 cell.
(B) Light-activated excitatory postsynaptic potentials (EPSPs) triggered by patterned illumination of a 10×10 grid with 473 nm LED.
(C) Colormap of the activation pattern.
(D) Histogram of automatically measured responses from all cells in a grid. Objective lens is 10X.
Courtesy Qiuyu Wu & Dr Alexander Chubykin, Purdue University, USA.
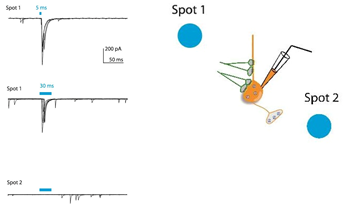
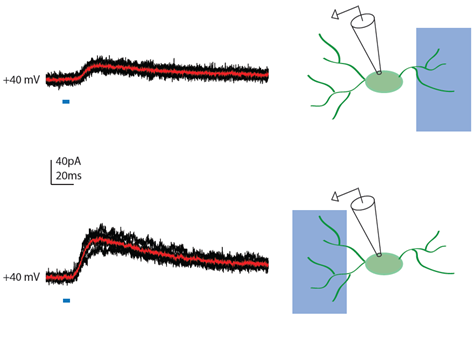
Optogenetic Stimulation of Synaptic Inputs
Light-evoked inhibitory postsynaptic current (IPSC) recorded in a transgenic mice expressing ChR2 in GABAergic neurons. The postsynaptic cell is non-GABAergic (ChR2 negative) and blue light stimulates GABAergic afferents expressing ChR2. Blue bars indicate the time of light illumination. Spot 1 illuminated by Polygon400 evoked reliable IPSCs whereas Spot 2 caused no response.
Courtesy Dr Wataru Inoue, University of Western Ontario, Canada.
Light-Modulated Protein-Protein Interactions on a Coverslip Surface
This video shows a protein-protein interaction that is inhibited by blue light exposure. One component is coupled to the coverslip through biotin surface chemistry, the other is labeled with mCherry, and binds to the coverslip in the dark. Patterned illumination with the Polygon400 results in revesible dissociation of the mCherry-tagged protein, which rebinds within minutes of turning off blue light exposure. The Wittmann lab is working on developing this into a cell adhesion surface that can be controlled by light.
Courtesy Dr Torsten Wittmann, University California San Francisco, USA.
Publications by this Customer using Polygon:
Mapping Optically Induced Depolarization in ChR2-expressing Hippocampal Neurons using Polygon400
E18 Sprague-Dawley rat neurons were transduced with CamKII-ChR2-GFP lentivirus. Somatic activity was recorded via whole-cell patch-clamp electrophysiology. Each field was illuminated by the Polygon400 at 100% power and 20ms exposure time. Intensity of magenta pattern represents depolarization with respect to the instantaneous resting potential prior to stimulation with the Polygon’s 470nm LED. In the image, green represents the magnitude of GFP signal and black represents the fluorescence intensity of AlexaFluor 594 backfilled by the patch pipette.
Courtesy Dr Jacob Robinson, Rice University, USA.
Publications by this Customer using Polygon:
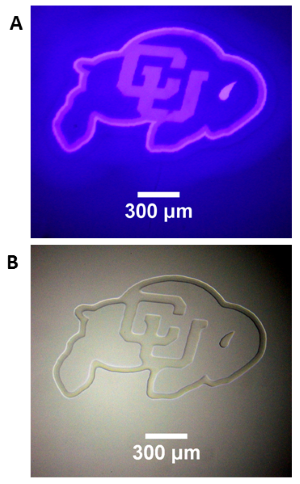
Photopatterning of an Acrylate Film
A 50 um film of liquid tetra (ethylen glycol) diacrylate, containg 1 wt% Irgacure 819 photointiator was irradiated through a glass coverslip, using the Polygon400 and a 400nm LED light source to project a pattern onto the film surface. (A) Projection of CU logo image using the 400nm LED and a 4X objective. (B) Brightfield image of the resulting pattern in the film. Photopolymerization causes a large change in refractive index in the resin, allowing immediate visualization of the pattern. Standard development techniques could subsequently be performed by washing the film in solvent to remove the unexposed areas of liquid resin.
Courtesy Gayla Berg, University of Colorado, USA.
Publications by this Customer using Polygon:
McBride et al. 2017 Advanced Materials
Konetski et al. 2016 Langmuir
Peng et al. 2014 Chemistry of Materials
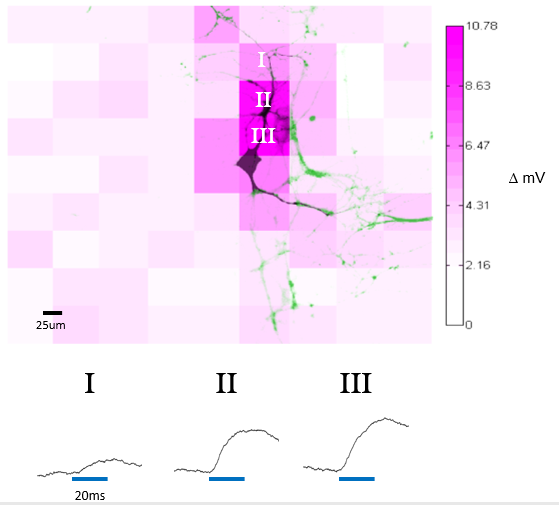
Achieving Subcellular Resolution of Input Activation in a 250um-thick Acute Cortical Section Using the Polygon400
NMDA-receptor mediated synaptic currents recorded at a +40mV membrane potential were elicited by light activation of channelrhodopsin-expressing terminals of thalamocortical afferents onto an upper layer cortical GABAergic interneuron. moving the 470nm rectangular illumination to the right or the left of the cell activates inputs of different strength innervating different somatodendritic domains of the cell. In black and red are the individual and the average traces respectively.
Courtesy Dr. Theofanis Karayannis, University of Zurich, Switzerland.
Publications by this Customer using Polygon:
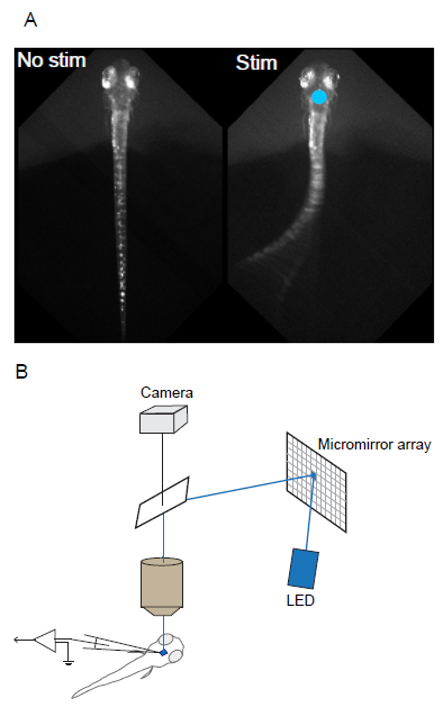
In Vivo Optogenetic Control of Zebrafish Larva Using Polygon400 Illuminator
A) Light-evoked response of a head-fixed larva expressing channelrhodopsin (right). Photostimulation site was indicated by a blue circle (470nm).
B) A schematic diagram of the Polygon400 patterned illumination in in vivo optogenetic mapping system.
Courtesy Dr. Sachiko Tsuda, Saitama University, Japan.
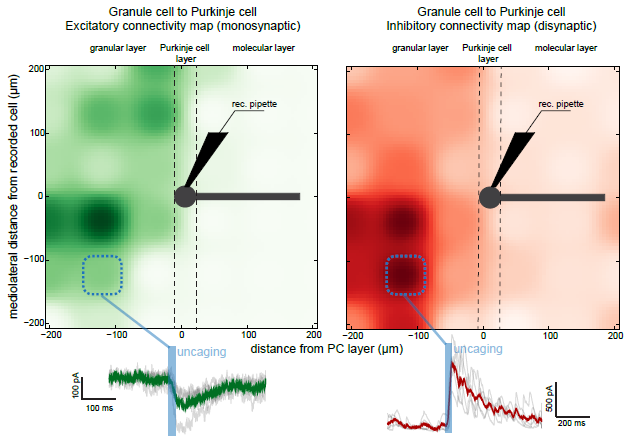
Uncaging RuBi Glutamate with the Polygon400 to Study Neuronal Networks in the Cerebellar Cortex
An example of connectivity mapping that allow to reproduce some results from in Valera et al. (elife, 2016). 100um RuBi glutamate was uncaged at various locations in the granular layer with 20ms pulses (blue bar), exciting notably granule cells. We can then measure the spatial organization of the granule cells to Purkinje cell (PC) connections by recording PCs in whole cell patch clamp. Granule cells triggers both monosynaptic excitatory current onto PCS (left map, measured a -60mW), but also disynaptic inhibitory currents via molecular layer interneurons (right map, measured at 0 mW). Average evoked responses at one location (dotted blue square) are shown at the bottom of the figure. Data storage, measurements, and map representations can be made online, using a homemade software in python.
Courtesy, Dr. Antoine Valera & Dr. Angus Silver, University College London, UK.
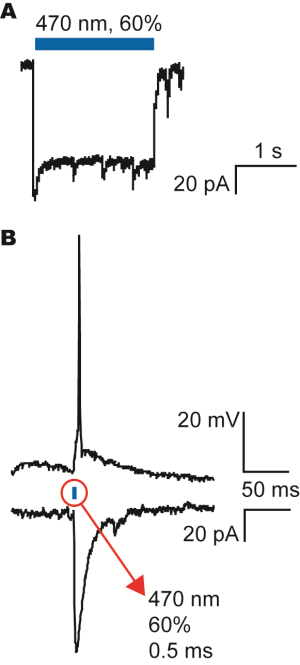
Optogenetic Activation of Channelrhodopsin in Transfected Hippocampal Neurons Using Mightex’s Polygon400
A) Patch-clamp recording of the current generated by the channelrhodopsin when activated with the blue LED light from the Polygon400 illuminator as indicated by the blue bar.
B) Activation of an action potential (upper trace) and the corresponding current under voltage-clamp conditions (lower trace). The action potential could be evoked by a 0.5ms stimulation of the blue LED light from the Polygon400 illuminator at 60% intensity.
Courtesy, Dr. Hans van Hooft, University of Amsterdam, Netherland.
Using Polygon400 to Stimulate Hippocampal Slices Acutely Prepared from Mice Transgenically Expressing ChR2 in the Dentate Gyrus
The first figure a repetitive stimulation with a larger circular pattern which elicited increasing responses until an action potential is generated. The second figure is increasing the intensity of stimulation from 30% to 100% and keeping the size of the pattern the same. Here we’re driving the cell at 10Hz at 100% and one can see that the stimulation is sufficient to drive doublets of action potentials during each bout of depolorization.
Courtesy, Dr. Geoffrey G. Murphy, University of Michigan, USA.
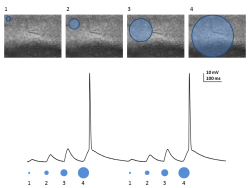
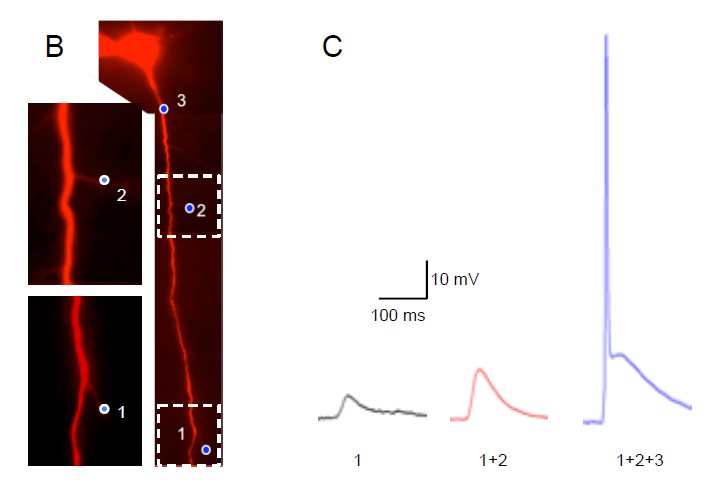
Local Photostimulation of Channelrhodopsin-2 Using Polygon400 Illuminator
A) Acute brain slice from a YFP-channelrhodopsin-2 (ChR-2) mouse depicting its expression in cortical L5 pyramidal neurons. B) L5 pyramidal neuron from the somatosensory cortex filled with Alexa 594 to allow visualization of neuronal compartments without stimulating ChR-2. Blue dots indicate on the illuminated areas (Blue LED – 470 nm) along the apical dendrite. Expansion of the marked areas depicting the delicate dendrites that were stimulated. C) Electrophysiological (patch clamp) current recordings from the soma, corresponding to local photostimulation of ChR-2 by blue light. The numbers in the bottom of the trace corresponds to the stimulated dot numbers as indicated in B.
Courtesy, Dr. Yossi Buskila, University of Western Sydney, Australia.
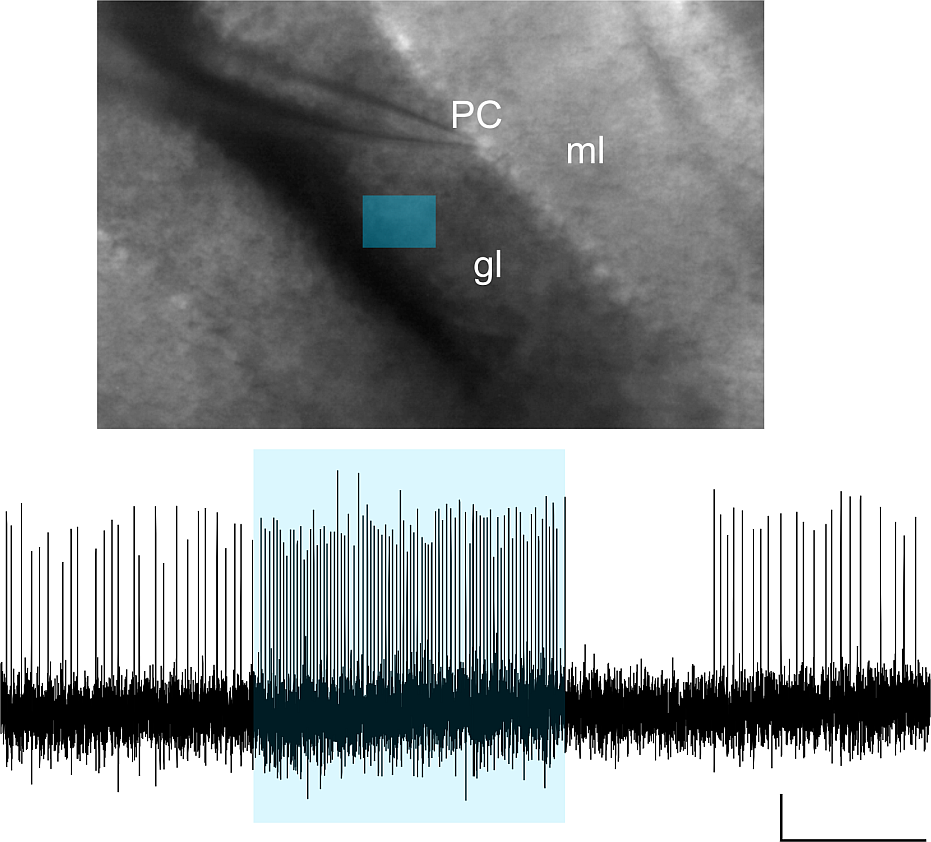
Purkinje Cell Firing Properties Unveiled by Optogenetic Activation of the Cerebellar Granular Layer Using Variable Light Patterns with Mightex Polygon400
Purkinje cell (PC) firing is monitored, while various light stimulation patterns are delivered to the granular layer (gl) and/or molecular layer (ml) of acute mouse cerebellar slices. The mice cerebellum was injected with AAVs carrying the ChR2 construct. The figure shows the firing frequency increase caused in a PC unit (scale bar 500ms) by optogenetic activation of a granular layer ROI (blue rectangle).
Courtesy, Dr. Lisa Mapelli & Dr. Simona Tritto, University of Pavia, Italy.
- (In Canada) 200 Consumers Road, Suite 805,
Toronto, Ontario M2J 4R4, CANADA - (Canada) 1-416-840-6115
- 1-416-840 6541
- (In US) 1241 Quarry Lane, Suite 105,
Pleasanton, CA 94566, USA - (US) 1-925-218-1885
- 1-416-840 6541



