Published on 2024/11/06 Research powered by Mightex’s Polygon1000 

This research was recently presented by Hassan Hosseini at the Society for Neuroscience (SFN 2024) in Chicago,USA and was supported by a Mightex Travel Award to promote scientific communication and sharing of research using the Mightex Polygon. Congratulations to Hassan Hosseini! We can’t wait to see more from this exciting project!


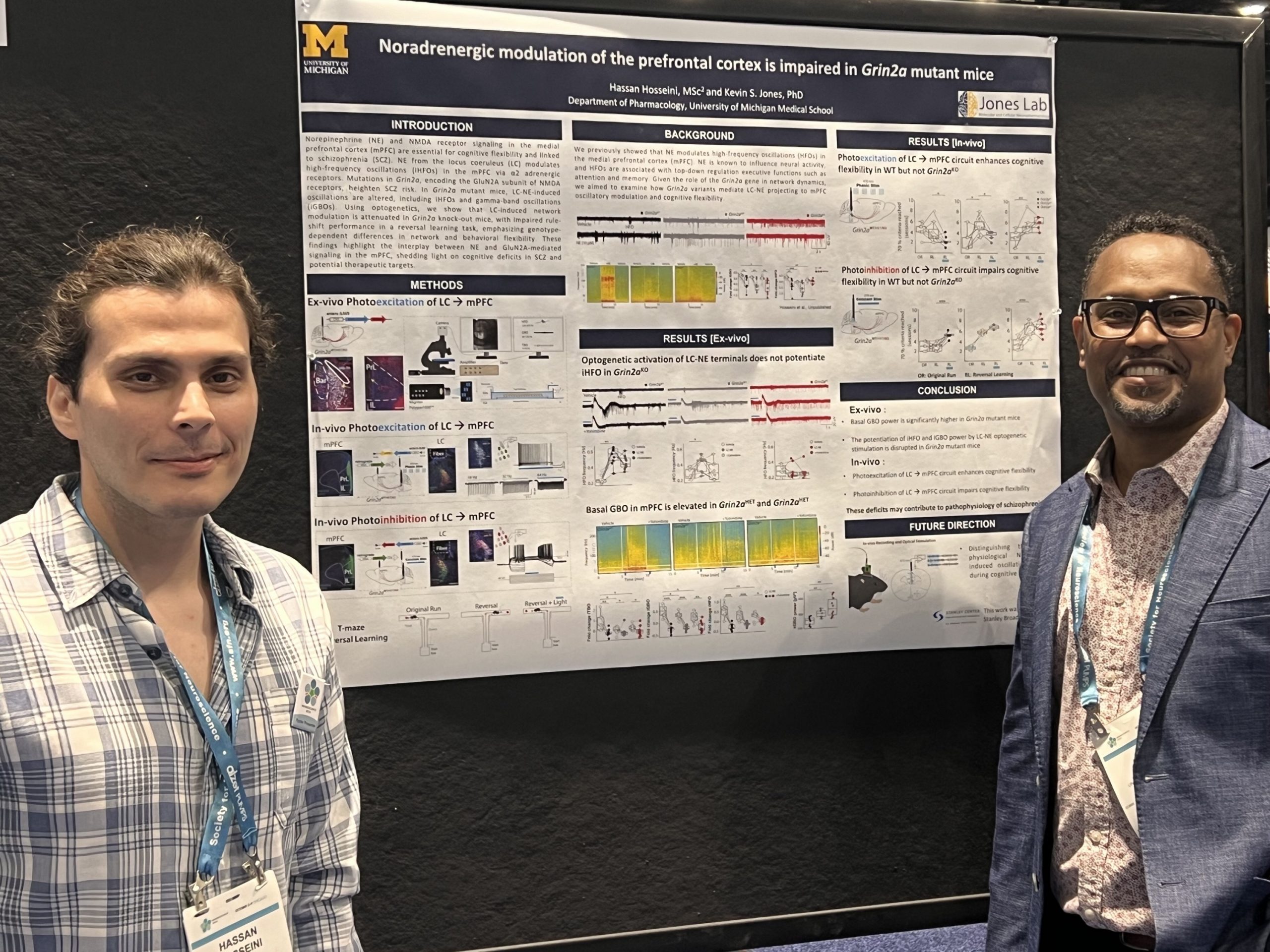
Schizophrenia is characterized by profound cognitive deficits, particularly in executive functions, which remain largely unaddressed by current pharmacological treatments. Recent research has implicated the GRIN2A gene—which encodes the GluN2A subunit of the N- methyl-D-aspartate receptor (NMDAR)—as a significant risk factor in schizophrenia. This finding suggests that loss-of-function mutations in GRIN2A may disrupt neural circuit functions crucial for cognition. The medial prefrontal cortex (mPFC) is a critical hub for cognition and is strongly modulated by noradrenaline (NE).
In this study, we utilized Grin2a mutant mice to explore the role of NE in mediating oscillatory activity within the mPFC. We employed optogenetic stimulation of locus coeruleus-noradrenergic (LC-NE) terminals in ex-vivo mPFC slices. We used planar multi-electrode array and patch-clamp electrophysiology to monitor the resultant neural activity, namely high-frequency oscillations (HFOs) and theta-gamma phase amplitude coupling (PAC). HFOs are rapid neural oscillations associated with cognitive processing and memory formation. Phase-amplitude coupling (PAC) is a mechanism thought to coordinate neural communication and cognitive processes across the brain, and theta-gamma phase-amplitude coupling (PAC) is crucial for coordinating cognitive processes. Aberrant HFOs and PAC, common in schizophrenia, likely reflect underlying neural disruptions. Aberrant HFOs and PAC are observed in schizophrenia patients, potentially reflecting the underlying disruptions in neural circuitry that contribute to cognitive deficits.
Our results indicate that NE significantly enhances HFO power in wild-type mice (98.3 %, SD: 35.0 %), compared to heterozygous and knockout Grin2a mutants (HET: 30.8%, SD: 27.1%; KO: 76.8%, SD: 23.8%; F=12.4, p=0.00022). Furthermore, NE’s role in strengthening PAC was found to be dependent on the function of GluN2A-containing NMDARs, a finding that we confirmed in WT tissue using subunit-selective NMDAR antagonists. Our findings suggest that disruptions in NE signaling and GluN2A functionality could underlie some of the cognitive deficits observed in schizophrenia, offering potential targets for therapeutic intervention.
This research advances our understanding of the neurophysiological mechanisms underlying schizophrenia and provides a foundation for the development of novel treatments aimed at restoring cognitive function in affected individuals.
This study used the Mightex Polygon1000 to target 470nm LED light in slice preparation in order to target activation of PV+ and SST+ interneurons in the mPFC.

Author: Hassan Hosseini
Bio: Research Laboratory Specialist Associate Intermediate, Department of Pharmacology, University of Michigan Medical School, Ann Arbor, MI. Advisor: Dr. Kevin Jones.