We are pleased to share the recent work of Md. Joynal Abedin, a postdoctoral researcher in the Berdichevsky Lab at LeHigh University, and a recent recipient of the Mightex Travel Award for their work using the Mightex Polygon for patterned optogenetics examination of seizure activity in slice preparation. Their findings offer a potential alternative, gentle yet effective means of suppressing seizures.
Introduction
The most prevalent refractory form of epilepsy is mesial temporal lobe epilepsy which involves seizures arising from hippocampus. An alternative option to surgical resection for treating patients with intractable epilepsy is to suppress seizures with electrical stimulation. Current stimulation protocols suppress seizures by delivering electrical pulses at high frequency, which effectively prevents activity in stimulated area. Md Joynal and colleagues were interested in developing an effective method for suppressing seizures in such a way that activity in stimulated region is not completely shut off during the intervention. This allowed stimulated area to participate in information processing and exchange of information with other brain regions, and may be a more gentle method of suppressing seizures than existing protocols.
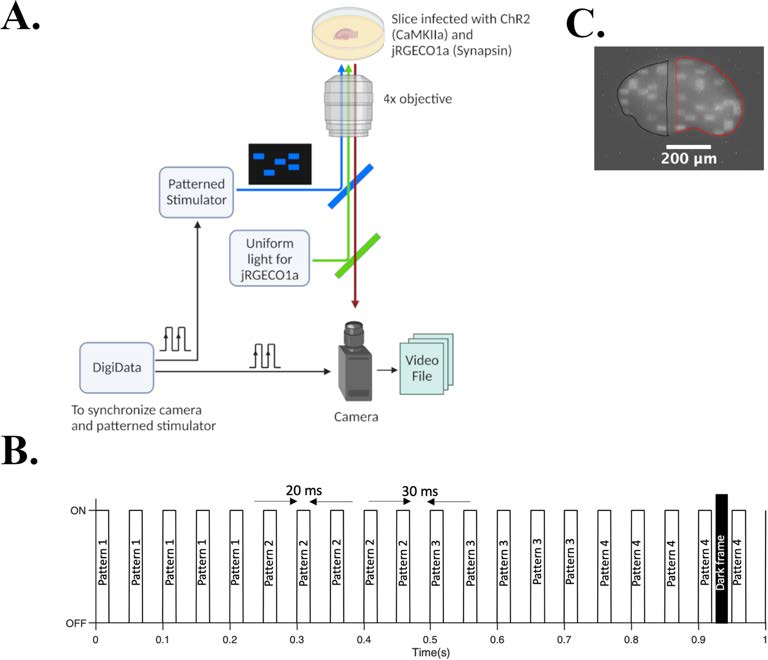
Figure 1: Pulsed Optogenetic Stimulation to whole slice to suppress seizure featuring Mightex Polygon-400 G.
(A) Experimental set up. DigiData synchronizes camera and patterned stimulator (Mightex polygon 400-G). (B) Timing diagram of the stimulation paradigm. Each of the 4 patterns repeated 5 times, and cyclically delivered. (C) Example of an input (inside black contour) and output pattern (inside red contour) being illuminated on the slice. One empty column of a 30Å~30 grid is separating the input and output pattern.
Experimental Design
During ictal activity, neurons in a network fire at high rate. Ictal episode terminates when neurons run out of neurotransmitter. Therefore, by keeping the synapses in the epileptic network depressed (short term depression) by delivering asynchronous stimulation, the authors sought to suppress seizures without shutting off all neural activity. They tested this hypothesis by delivering spatially patterned optical pulses to rat (post-natal day 7/8) organotypic hippocampal slice cultures expressing channel rhodopsin (ChR2), a blue light activated cation channel in excitatory neurons under CaMKII promoter. Slices also expressed jRGECO1a, a red fluorescent protein sensitive to intracellular calcium under synapsin promoter to enable us to monitor ictal-like activity. We developed a protocol to deliver patterned optogenetic stimulation to the whole slice. Recordings were 60 minutes long in total (20 mins each for spontaneous-with stimulationspontaneous recording). Mightex’s Polygon 400-G Pattern Illuminator for delivering stimulation, a CCD camera and a 4X objective were used to record fluorescent changes at 20 frames per second (Fig. 1). Videos were then analyzed to extract raw mean grey value using ImageJ and fluorescent change, ΔF over baseline, F was calculated in MATLAB using asymmetric least square mean smoothing method.
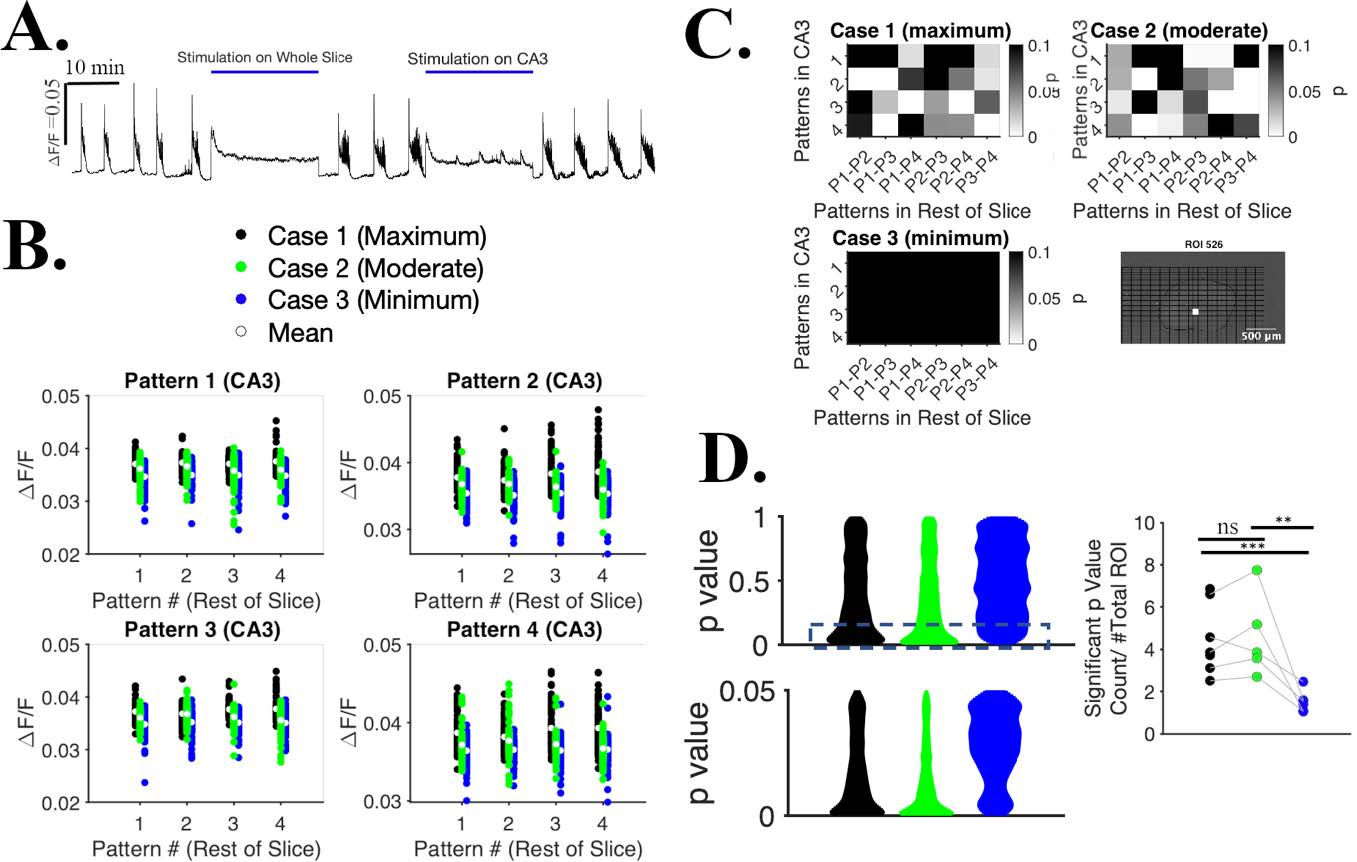
Figure 2: Pulsed optogenetic stimulation (using Mightex polygon 400-G) of organotypic
slice suppress seizures and allows information processing.
(A) ΔF/F from the whole slice sampled at the frequency of dark frames. (B) ΔF/F from ROI # 526, stimulating the slice with 3 protocols. (C) For each pattern in CA3, there are 6 combinations of comparison from 4 patterns in rest of slice. 24 p values in total, paired t-test for Case 1, 2 and 3. Location of the ROI# 526 shown in bottom right. (D) Distribution of significant p value count of a slice ( p=< 1) (top left), distribution of significant p value count of a slice ( p<0.05) (bottom left). Significant p value count normalized to # total ROIs across all slices, n= 7 slices for case 1 & 3, n=5 slices for case 2 (right). Two sampled ks-test, p= 1 (between Case 1 & 2), p=0.0004 (between case 1 & 3), p=0.002 (between case 2 & 3). ns: non significant.
Results and Conclusion
Spontaneous recordings show seizures (defined as at least 10 sec long activity above threshold) whereas seizures are abolished completely when whole slice is stimulated (Fig. 2 A). With CA3 stimulation, we observed that seizures appearance rate is lower, and seizure duration is shorter as well (Fig. 2 A). Once we suppress the seizure, we designed another set of 3 protocols where stimulation on CA3 was spatially same for all cases with (1) maximum stimulation spots on rest of slice (Fig. 1 C), (2) fewer stimulation spots on rest of slice, and (3) no stimulation spots on rest of slice. They kept the same spatial CA3 stimulation across protocols yet varied the input patterns in rest of slices 4 times for each of 4 patterns in CA3 (Fig. 2 B, C). We report that both case 1 and case 2 can distinguish variations of input patterns compared to case 3, two sampled ks-test, p=0.0004 (between case 1 & 3), p=0.002 (between case 2 & 3) (Fig. 2 D). Therefore, this protocol is ‘gentle’ enough that slice is not tetanized during stimulation and slice is able to process other inputs during stimulation.
These findings provide an alternative, gentle yet effective means of suppressing seizures. This method may improve the quality of life for epileptic patients by reducing side effects of neural stimulation.



