 Lee, J., Lai, S., Yang, S., Zhao, S., Blanco, F. A., Lyons, A. C., … & St-Pierre, F.,Bright and photostable yellow fluorescent proteins for extended imaging.
Lee, J., Lai, S., Yang, S., Zhao, S., Blanco, F. A., Lyons, A. C., … & St-Pierre, F.,Bright and photostable yellow fluorescent proteins for extended imaging.
Nature Communications, 16(1), 3241 (2025).
 Miyazaki, I., Tsao, K. K., Kamijo, Y., Nasu, Y., Terai, T., & Campbell, R. E.,Synthesis and application of a photocaged-l-lactate for studying the biological roles of l-lactate.Communications Chemistry, 8(1), 104 (2025).
Miyazaki, I., Tsao, K. K., Kamijo, Y., Nasu, Y., Terai, T., & Campbell, R. E.,Synthesis and application of a photocaged-l-lactate for studying the biological roles of l-lactate.Communications Chemistry, 8(1), 104 (2025).

Jihwan “James” Lee
Rice University, USA

Prof. François St-Pierre
Rice University, USA

Ikumi Miyazaki
Université Laval, Canada

Prof. Robert E. Campbell
Université Laval, Canada
Recent breakthroughs in optogenetics and live-cell imaging have unlocked exciting possibilities for probing cellular processes with exquisite spatiotemporal control. Two new studies — one focused on photoreleasable metabolic tools and another on advanced fluorescent proteins — showcase the power of photoactivation, with the Mightex Polygon digital micromirror device (DMD) playing a critical role in precise targeted illumination.
In the first study by Miyazaki et al. (Communications Chemistry, 2025), researchers synthesized a novel photocaged version of L-lactate (MNI-L-lac) to explore this important molecule’s signaling and metabolic roles. Long dismissed as a metabolic byproduct, L-lactate is now recognized for its signaling functions, including modulation of the GPR81 receptor and regulation of cAMP levels. The authors demonstrated that upon light activation, MNI-L-lac efficiently releases L-lactate, which then engages both extracellular and intracellular biosensors (eLACCO2.1 and R-iLACCO1.2, respectively, Figure 1). Notably, photoactivation was performed using a 405 nm light pulse delivered via the Mightex Polygon, allowing for spatially resolved uncaging during real-time imaging of cellular responses.
In parallel, Lee et al. (Nature Communications, 2025) tackled a longstanding limitation in live-cell imaging: the rapid photobleaching of yellow fluorescent proteins (YFPs). They engineered mGold2s and mGold2t — YFPs with dramatically enhanced photostability (up to 25× that of mVenus) while maintaining brightness, all through a high-throughput single-cell screening enabled by the Mightex Polygon.
Together, these studies exemplify the synergy between chemical biology and optical engineering. In the first study, precise targeting of light was essential to control when and where L-lactate was released. In the second study, the Mightex Polygon restricted light to a 4 μm × 4 μm region centered on the target cells.
In summary, these innovations expand the toolkit for probing cell physiology and underscore the Mightex Polygon’s growing role in modern optobiology labs.

Figure 1: Uncaging of MNI-L-lac with the Mightex Polygon using 405nm illumination. L-lactate biosensor reports increased intracellular concentration after uncaging.
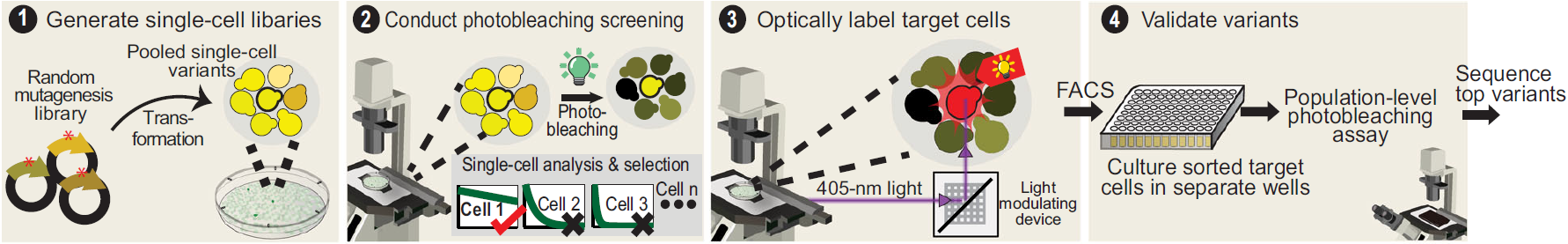
Figure 2: High-throughput single-cell screening used to engineer more photostable YFP constructs. The Mightex Polygon DMD optically tags resistant cells.
Francisco Rodrigues, PhD, Manager, Customer Solutions at Mightex
To read the full publication, please click here (Lee at al) or here (Miyazaki et al)












