Published on 2018/08/17 Research powered by Mightex’s Polygon1000 
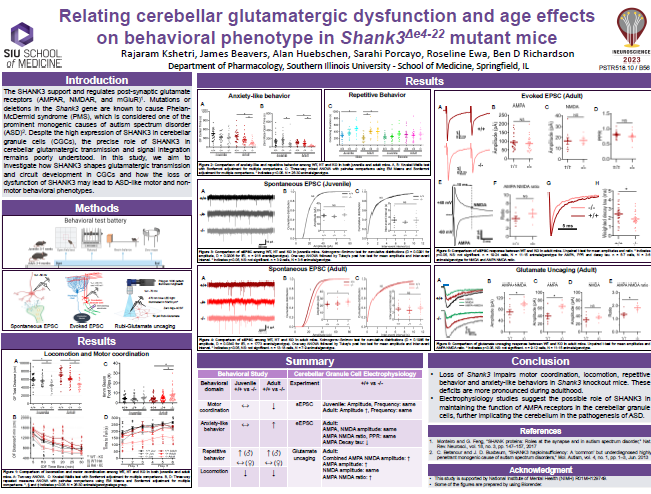
Rajaram Kshetri & Ben Richardson, Relating cerebellar glutamatergic dysfunction and age effects on behavioral phenotype in Shank3△e4-22 mutant mice. Southern Illinois University, (2023).


Post-synaptic glutamate receptors (AMPAR, NMDAR, and mGluR) are supported and regulated by the SHANK3 scaffold protein (Shank3 gene), deletions/mutations in which are a prominent monogenic cause of autism spectrum disorder (ASD). Despite high SHANK3 expression in cerebellar granule cells (CGCs), the primary integrator of cerebellar input, the role of SHANK3 in cerebellar glutamatergic transmission and signal integration is poorly understood. We hypothesize that SHANK3 shapes the nature of glutamatergic transmission and circuit development in CGCs, the loss or dysfunction of which may result in ASD-like motor and non- motor behavioral phenotypes. The purpose of this study is to understand how the loss of SHANK3 affects the function and structure of glutamatergic CGC synapses that may affect cerebellar modulation of motor and non-motor behaviors. In order to identify gene-, sex-, and age-related interactions and main effects on behavioral phenotype, adolescent (5-7 weeks old) and adult (3-5 months old) mice of both sexes carrying the wildtype Shank3 gene (Shank3+/+)or that were heterozygous (Shank3+/-) or homozygous (Shank3-/-) for a version of the Shank3 gene lacking exons 4-22 (all isoforms) were used in a behavioral battery to assess motor function, anxiety, repetitive behavior, memory, and social interaction. We found more prominent Shank3 genotypic differences in motor function, increased anxiety, and repetitive behavior in adult mice compared to adolescent mice.
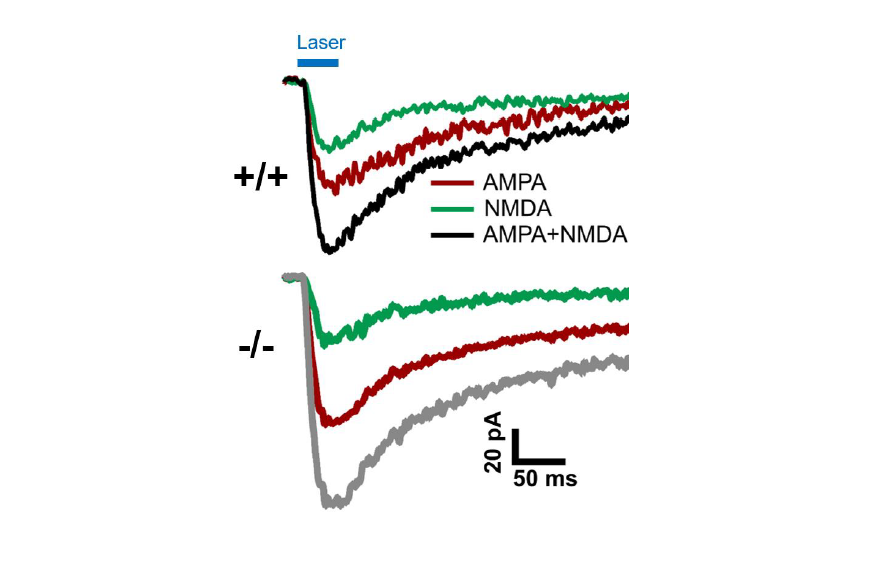
Spontaneous and pharmacologically-evoked glutamate receptor-mediated responses in CGCs were evaluated by whole-cell patch clamp electrophysiology and glutamate photo-uncaging. Our preliminary electrophysiological findings suggest a parallel relationship between the age-related increase in behavioral deficits and the enhancement of spontaneous excitatory postsynaptic currents (sEPSCs) amplitude in CGCs of adult Shank3-/- mice. Faster decay kinetics were observed in the evoked excitatory postsynaptic currents (eEPSCs) of Shank3-/- mice, in comparison to Shank3+/+ mice. Ongoing glutamate photo-uncaging experiments in mature adult mice showed an increased AMPA/NMDA ratio and enhanced responsivity of glutamate receptors (AMPA and NMDA combined) in CGCs of Shank3-/- mice, relative to Shank3+/+ mice. These findings suggest the possible role of SHANK3 in maintaining glutamatergic receptors and synapses in CGCs, as well as the potential involvement of the cerebellum in ASD. These insights may underlie and present a novel treatment target for motor and certain cerebellum-modulated non-motor behavioral deficits observed in the absence of Shank3 activity or function.
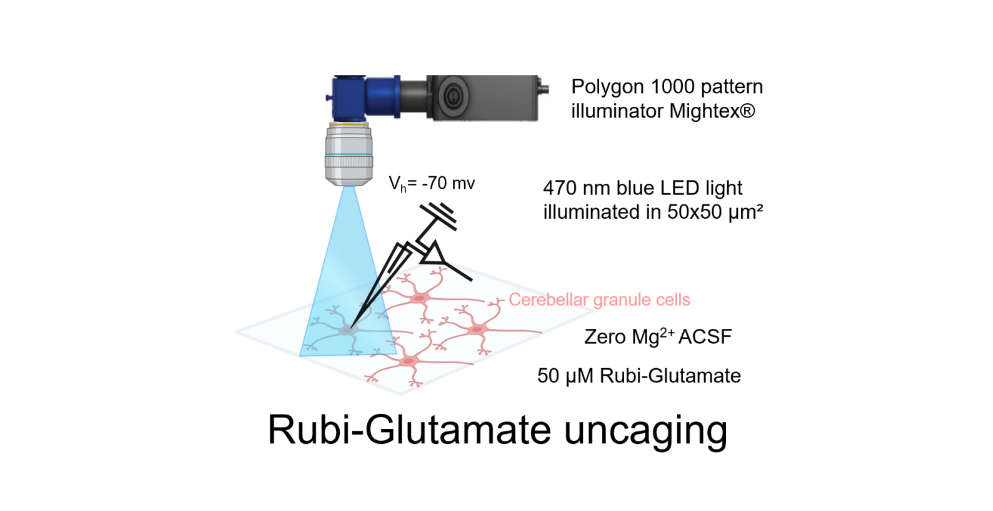
For the glutamate photo-uncaging experiment, we used Ruthenium-bipyridine-trimethylphosphine-Glutamate (RuBi-Glutamate), which is a caged glutamate compound. This compound can be excited by visible wavelengths and releases glutamate upon one- or two- photon excitation. CGC was maintained at a holding potential of -70 mV, and the photo-evoked EPSCs were recorded in the whole-cell configuration. Focal stimulation of CGC was achieved by applying 470 nm blue LED light within a 50×50 μm2 gridded area over the cerebellar cortex. To facilitate photo-release of glutamate in the specified area, the Mightex Polygon 1000 pattern Illuminator was used. This experiment was conducted in the presence of 50 μM RuBi-Glutamate, chosen as the optimal concentration based on reliable response using the stimulation protocol. Glutamate photo-release was achieved by using a light stimulation duration of 50 ms at a frequency of 0.05 Hz. The recording setup involved perfusion of the bath with zero Mg2+ ACSF to record both AMPA and NMDA responses at a membrane potential of -70 mV, using a CsCl-based internal solution. To record the combined AMPA+NMDA current evoked by photo-stimulation, the cerebellum slice was perfused with RuBi-Glutamate along with gabazine (10 μM) and tetrodotoxin (0.5 μM). For recording NMDA current, the bath was supplemented with 10 μM NBQX. The NMDA current was validated by subsequent bath application of 50 μM AP5 and 50 μM 7-Chlorokynurenic acid.
Author: Rajaram Kshetri, PhD Candidate
Bio: Ph.D. Candidate-Department of Pharmacology, Southern Illinois University, School of Medicine, Springfield, IL, USA
2014 Bachelor of Pharmacy (B. Pharm.)-Tribhuvan University, Kathmandu, Nepal



