
Neuronal sensorimotor integration guiding salt concentration
navigation in Caenorhabditis elegans
navigation in Caenorhabditis elegans
Published on 2024/04/02 Research powered by Mightex’s Polygon1000 



Introduction
In order to properly navigate our environment, we integrate sensory information (what we see, what we smell, what we hear, etc.) with the movements we make. How these different types of information are combined in order to produce appropriate outcome behaviours is complex and depends on many contextual factors. The mechanisms underlying this process are largely unknown.
C. elegans use chemotaxis to move towards different water soluble chemicals, such as sodium chloride (NaCL; Bargmann & Horvitz, 1991). They are able to sense NaCl concentration gradients using unique chemosensory receptors (ASEL and ASER) located on the anterior part of their body. When they have been exposed to food with high salt concentration, C. elegans will go on to show appetitive behaviour towards areas of high salt concentration, using dorsoventral movements of the anterior portion of their body (Kunitomo et al., 2013). They do so by creating a sensory map of the salt concentration differences that cross their path at a perpendicular angle to their direction of travel. They will then move towards areas which they sense to be higher in salt concentration by bending their head in a process called klinotaxis (Iino & Yoshida, 2009).
Klinotaxis requires the integration of temporal changes in sensory signals with feedback from motor neurons in order to guide subsequent movement towards locations of higher chemical concentrations. How these sensory and motor signals are integrated is not fully known. Matsumoto and colleagues propose that, in C. elegans, the ASER neuron is responsible for NaCl sensing, while the SMBD motor neuron is responsible for neck bending. In this paper, Matsumoto and colleagues used a combination of behavioural analysis, neural response, and optogenetic perturbation experiments in order to interrogare their proposed sensorimotor signal integration model. In particular, the authors employed patterned optogenetics using the Mightex Polygon to sculpt chemotaxis behaviour, adding support for their proposed mechanism of signal integration.
Results
In order to artificially generate salt concentrations and allow recording of head bending direction, Matsumoto and colleagues developed a “head-swing chip” microfluidic device in which the C. elegans were able to freely move their heads while the remainder of their body was fixed in place. A y-shaped inlet design then allows sensing of different NaCl concentrations in a spatiotemporal manner. They found that neck bending towards higher NaCl concentration was observed in a phase-dependent manner. That is, when NaCl gradient was higher, neck bending favoured ipsilateral movement whereas when NaCl was lower, neck bending favoured contralateral movement.
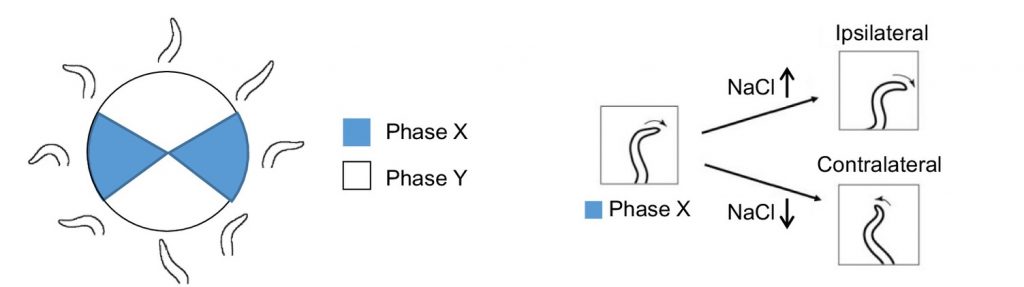
Figure 1: Schematic of phase-dependent neck bending in “head-swing chip”
The authors also found that this NaCl concentration sensing was inversely related to ASER neuronal activity, as measured by calcium imaging. As NaCl concentration increased, activity of ASER neuron decreased, whereas as NaCl concentration decreased, activity of the ASER neuron increased. Furthermore, activity of the ASER neuron was robustly correlated to neck bending direction such that ventral head bending was associated with SMBD activity, while dorsal neck bending was associated with SMBD quiessence.
In order to examine how signal from these sensory and motor neurons integrate in order to produce environmentally appropriate behaviour, Matsumoto and colleagues employed the Mightex Polygon to produce patterned optogenetics to interrogate the impact of SMBD neuronal activation during ventral neck movements in changing NaCl concentration environments.
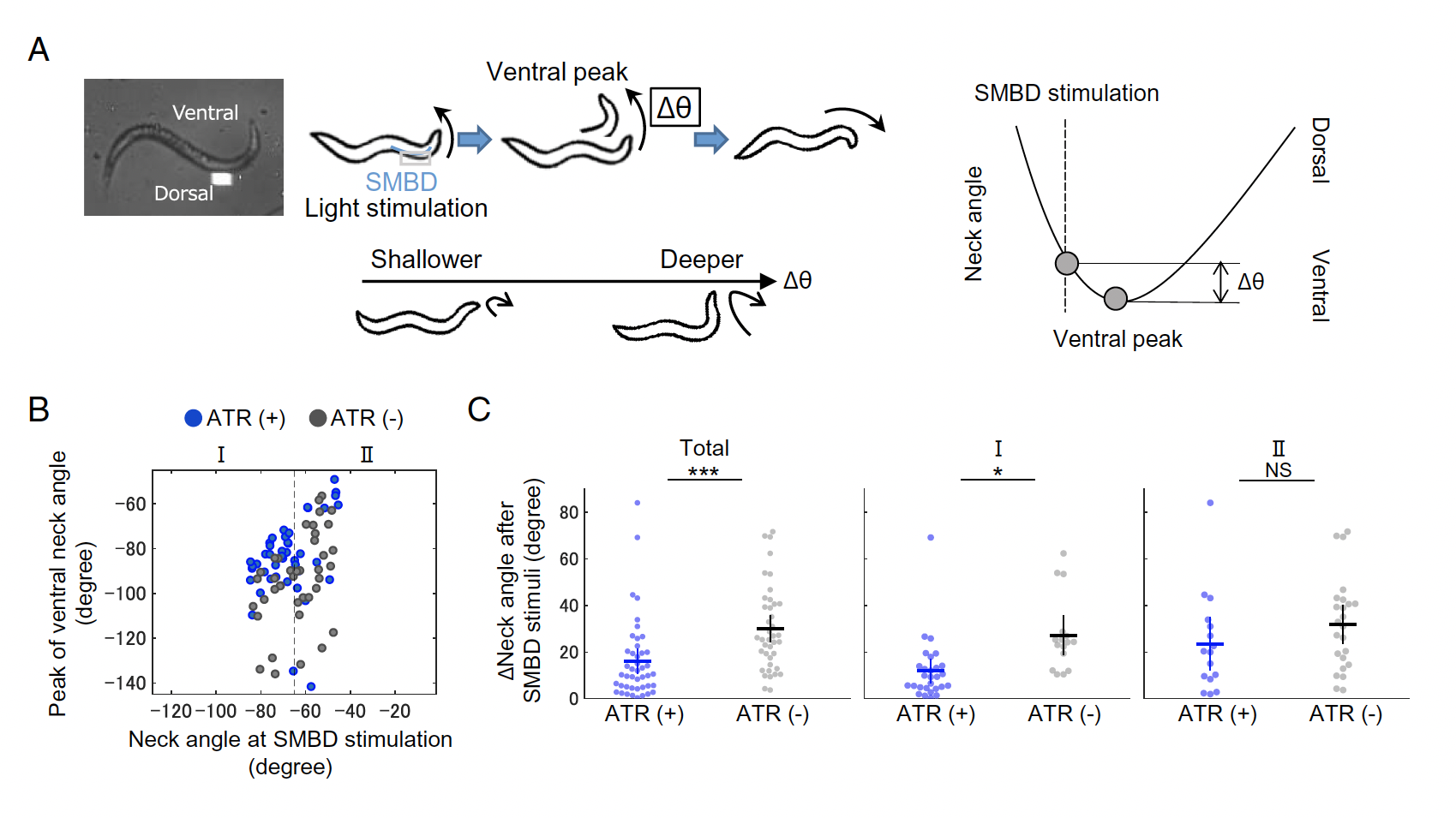
Figure 2: Mightex Polygon for patterned optogenetics interrogating sensorimotor signal integration in klinotaxis. A) Schematic of patterned stimulation of SMBD neuron. B) Impact of ChR2 SMBD activation on neck bending degree.
Using ChR2, 470nm LED light (Mightex Systems), and patterns generated using Mightex’s PolyScan2 software, the authors stimulated EMBS activity during ventral neck movement, finding that intense SMBD activation resulted in subsequently shallower ventral neck movements. This suggests that sensing decreasing NaCl while experiencing strong ventral neck bending results in a surge of SMBD activation and compromised future klinotaxic behaviour.
These findings, together with computational simulation work of the authors, support Matsumoto and colleagues’ proposed mechanism of C. elegans klinotaxic sensorimotor integration via the ASER and SMRB neurons.
References:
Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991 Nov;7(5):729-42. doi: 10.1016/0896-6273(91)90276-6. PMID: 1660283.
Kunitomo H, Sato H, Iwata R, Satoh Y, Ohno H, Yamada K, Iino Y. Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt concentration chemotaxis in Caenorhabditis elegans. Nat Commun. 2013;4:2210. doi: 10.1038/ncomms3210. PMID: 23887678.
Iino Y, Yoshida K. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J Neurosci. 2009 Apr 29;29(17):5370-80. doi: 10.1523/JNEUROSCI.3633-08.2009. PMID: 19403805; PMCID: PMC6665864.
Catherine Thomas, PhD Senior Liaison and Development Scientist at Mightex
To read the full publication, please click here.