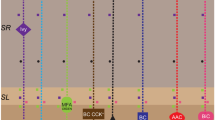
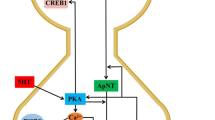
Heterosynaptic plasticity is a poorly studied type of synaptic plasticity in which synapses not active during the plasticity induction process are modified. The main methodological problem in studying the mechanisms of heterosynaptic plasticity using conventional techniques are the need for large numbers of experiments because of the probabilistic nature of the development of plastic changes in the synaptic inputs to neurons after intracellular tetanization – the most widely used approach to inducing heterosynaptic plasticity. To overcome this problem, we report here use of a method of delivering optogenetic stimulation to a multitude of presynaptic neurons converging on a single postsynaptic neuron. Our studies using rat brain slices showed that intracellular tetanization of a pyramidal neuron in layer 5 of the neocortex leads to the development of depression or potentiation of a proportion of the synaptic inputs from above-lying pyramidal cells in layers 2/3 expressing light-activated protein channelrhodopsin-2. Use of this stimulation method was found to allow a single experiment to yield sufficient data for statistical evaluation of the correlation between the paired pulse ratio and the amplitude of changes in EPSP after intracellular tetanization. We also showed that so-called “silent” synapses were not activated on development of long-term potentiation induced by intracellular tetanization.
Similar content being viewed by others
References
Baker, C. A., Elyada, Y. M., Parra, A., and Bolton, M. M., “Cellular resolution circuit mapping with temporal-focused excitation of soma-targeted channelrhodopsin,” eLife, 5, e14193 (2016).
Chen, J. Y., Lonjers, P., Lee, C., Chistiakova, M., et al., “Heterosynaptic plasticity prevents runaway synaptic dynamics,” J. Neurosci., 33, 15915–15929 (2013).
Hebb, D. O., The Organisation of Behavior, Wiley, New York (1949).
Hernández-Miranda, L. R., Parnavelas, J. G., and Chiara, F., “Molecules and mechanisms involved in the generation and migration of cortical interneurons,” ASN Neuro., 2, No. 2, e00031 (2010).
Ilin, V., Malyshev, A., Wolf, F., and Volgushev, M., “Fast computations in cortical ensembles require rapid initiation of action potential,” J. Neurosci., 33, 2281–2292 (2013).
Kerchner, G. A. and Nicoll, R. A., “Silent synapses and the emergence of a postsynaptic mechanism for LTP,” Nat. Rev. Neurosci., 9, 813–825 (2008).
Kuhnt, U., Kleschevnikov, A. M., and Voronin, L. L., “Long-term enhancement of synaptic transmission in the hippocampus after tetanization of single neurones by short intracellular current pulses,” Neurosci. Res. Comm., 14, 115–123 (1994).
Lee, C. M., Stoelzel, C., Chistiakova, M., and Volgushev, M., “Heterosynaptic plasticity induced by intracellular tetanization in layer 2/3 pyramidal neurons in rat auditory cortex,” J. Physiol., 590, No. 10, 2253–2271 (2012).
Letzkus, J. J., Kampa, B. M., and Stuart, G. J., “Learning rules for spike timing-dependent plasticity depend on dendritic synapse location,” J. Neurosci., 26, 10420–10429 (2006).
Löwel, S. and Singer, W., “Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity,” Science, 255, 209–212 (1992).
Maher, B. J. and LoTurco, J. J., “Disrupted-in-schizophrenia (DISC1) functions presynaptically at glutamatergic synapses,” PLoS One, 7, No. 3, e34053 (2012).
Malyshev, A. Y. and Balaban, P. M., “Synaptic facilitation in Helix neurons depends upon postsynaptic calcium and nitric oxide,” Neurosci. Lett., 261, 65–68 (1999).
Petreanu, L., Huber, D., Sobczyk, A., and Svoboda, K., “Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections,” Nat. Neurosci., 10, No. 5, 663–668 (2007).
Reyes, A. and Sakmann, B., “Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex,” J. Neurosci., 19, 3827–3835 (1999).
Sjöström, P. J. and Häusser, M. A., “Сooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons,” Neuron, 51, 227–238 (2006).
Thomson, A. M. and Bannister, A. P., “Postsynaptic pyramidal target selection by descending layer III pyramidal axons: dual intracellular recordings and biocytin filling in slices of rat neocortex,” Neuroscience, 84, 669–683 (1998).
Timofeev, I., “Neuronal plasticity and thalamocortical sleep and waking oscillations,” Prog. Brain Res., 193, 121–144 (2011).
Tononi, G. and Cirelli, C., “Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration,” Neuron, 81, 12–34 (2014).
Tononi, G. and Cirelli, C., “Sleep function and synaptic homeostasis,” Sleep Med. Rev., 10, 49–62 (2006).
Tononi, G. and Cirelli, C., “Time to be SHY? Some comments on sleep and synaptic homeostasis,” Neural Plast., 2012, Art. ID415250 (2012).
Volgushev, M., Balaban, P., Chistiakova, M., and Eysel, U. T., “Retrograde signalling with nitric oxide at neocortical synapses,” Eur. J. Neurosci., 12, 4255–4267 (2000).
Volgushev, M., Voronin, L. L., Chistiakova, M., and Singer, W., “Induction of LTP and LTD in visual cortex neurones by intracellular tetanisation,” Neuroreport, 5, 2069–2072 (1994).
Volgushev, M., Voronin, L. L., Chistiakova, M., and Singer, W., “Relations between long-term synaptic modifications and paired-pulse interactions in the rat neocortex,” Eur. J. Neurosci., 7, 1751–1760 (1997).
Von der Malsburg, C., “Self-organization of orientation sensitive cells in the striate cortex,” Kybernetik, 14, 85–100 (1973).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 67, No. 5, pp. 75–85, September–October, 2017.
Rights and permissions
About this article
Cite this article
Simonova, N.A., Bal, N.V., Balaban, P.M. et al. An Optogenetic Approach to Studies of the Mechanisms of Heterosynaptic Plasticity in Neocortical Neurons. Neurosci Behav Physi 49, 208–215 (2019). https://doi.org/10.1007/s11055-019-00716-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-019-00716-0